
Concept explainers
(a)
Interpretation:
The product should be identified when 1-ethylcyclohexene treated with
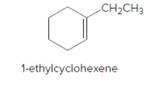
Concept Introduction:
Cyclic
Reaction of an alkene with
Answer to Problem 13.63P
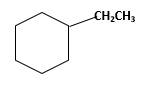
Explanation of Solution
Unsaturated 1-ethylcyclohexene,alkene molecule reacts with
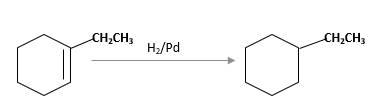
(b)
Interpretation:
The product should be identified when 1-ethylcyclohexene treated with
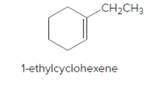
Concept Introduction:
Cyclic alkenes are hydrocarbon molecules that consist of a carbon-carbon double bond which has the general formula of
Reaction of an alkene with
Answer to Problem 13.63P
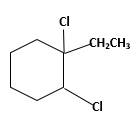
Explanation of Solution
Unsaturated 1-ethylcyclohexene, alkene molecule reacts with
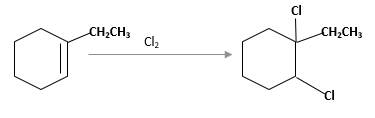
(c)
Interpretation:
The product should be identified when 1-ethylcyclohexene treated with
Concept Introduction:
Cyclic alkenes are hydrocarbon molecules that consist of a carbon-carbon double bond which has the general formula of
Reaction of an alkene with
Answer to Problem 13.63P
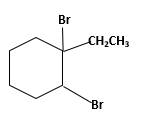
Explanation of Solution
Unsaturated 1-ethylcyclohexene, alkene molecule reacts with
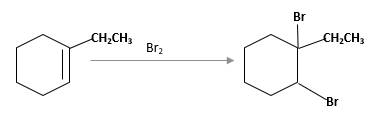
(d)
Interpretation:
The product should be identified when 1-ethylcyclohexane treated with
Concept Introduction:
Cyclic alkenes are hydrocarbon molecules that consist of a carbon-carbon double bond which has the general formula of
Reaction of an alkene with
Hydro halogenation reaction of alkenes follows the Markovnikov's rule.
Answer to Problem 13.63P
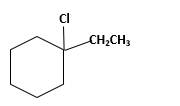
Explanation of Solution
Unsaturated 1-ethylcyclohexene, alkene molecule reacts with
Hydro halogenation reaction of alkenes follows the Markovnikov's rule. When
Refer to the below reaction:
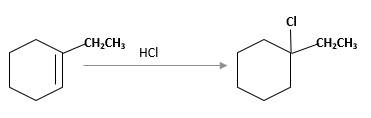
(e)
Interpretation:
The product should be identified when 1-ethylcyclohexene treated with
Concept Introduction:
Cyclic alkenes are hydrocarbon molecules that consist of a carbon-carbon double bond which has the general formula of
Reaction of an alkene with
Hydro halogenation reaction of alkenes follows the Markovnikov's rule.
Answer to Problem 13.63P
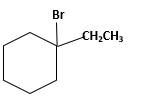
Explanation of Solution
Unsaturated 1-ethylcyclohexene, alkene molecule reacts with
Hydro halogenation reaction of alkenes follows the Markovnikov's rule. When
Refer to the below reaction;
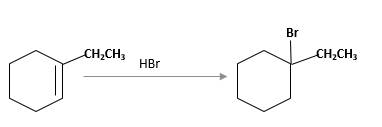
(f)
Interpretation:
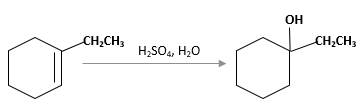
The product should be identified when 1-ethylcyclohexene treated with
Concept Introduction:
Cyclic alkenes are hydrocarbon molecules that consist of a carbon-carbon double bond which has the general formula of
Reaction of an alkene with
Hydration reaction of alkenes follows the Markovnikov's rule.
Answer to Problem 13.63P
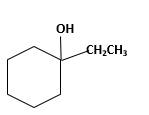
Explanation of Solution
Unsaturated 1-ethylcyclohexene, alkene molecule reacts with
Want to see more full solutions like this?
Chapter 13 Solutions
ALEKS 360 ACCESS CARD F/GEN. ORG.CHEM
- no AI walkthrough current image is wrong answerarrow_forwarda. Determine whether each of the Followery Molecules is in the R- On the y- Configuration 1-01"/ 1-6-4 Br 4 I el Br b. Draw The Fisher projection For all the Meso compounds that can exist FOR The Following molenlearrow_forward1- Refer to the monosaccharides below to answer each of the following question(s): CH₂OH CHO CH₂OH CH₂OH 0 H- OH 0 0 HO- H H- -OH HO H HO H H OH HO- H CH₂OH H. OH HO H HO- H CH₂OH CH₂OH CH3 a. Sorbose b. Rhamnose c. Erythrulose d. Xylulose Classify each sugar by type; for example, glucose is an aldohexose. a. Xylulose is .. b. Erythrulose is . c. Sorbose is .. d. Rhamnose is .. 2- Consider the reaction below to answer the following question(s). CHO H OH CH₂OH CH₂OH HO- H HO HO + H. -OH HO OH HO. H OH OH H -OH H OH CH₂OH Q Z a. Refer to Exhibit 25-11. Place a triangle around the anomeric carbon in compound Q. Compound Z is: b. 1. the D-anomer. 2. the a-anomer. 3. the ẞ-anomer. 4. the L-anomer. c. Which anomer is the LEAST stable? d. Q and Z are cyclic examples of: a. acetals b. hemiacetals c. alditols d. hemialditolsarrow_forward
- i need help identifying the four carbon oxygen bonds in the following:arrow_forwardImagine each of the molecules shown below was found in an aqueous solution. Can you tell whether the solution is acidic, basic, or neutral? molecule HO H3N + The solution is... X O acidic OH O basic H3N-CH-C-O O neutral ○ (unknown) O acidic ○ basic CH2 CH 3-S-CH2 O neutral ○ (unknown) H3N O OH O acidic O basic Oneutral O (unknown) 0 H3N-CH-C-O CH3 CH CH3 O acidic O basic O neutral ○ (unknown) ? olo Ar BHarrow_forwardno Ai walkthroughs need other product (product in picture is wrong dont submit the same thing)arrow_forward
- I have a 2 mil plastic film that degrades after 22 days at 88C and at 61C takes 153 days. What is the failure at 47C in days.arrow_forwardIf a 5 film plastic film degraded in 30 days at 35C and the same film degraded in 10 days at 55 C and 2 days at 65C what would the predicted life time be at 22C for the same film?arrow_forwardno Ai walkthroughsarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
