
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
5th Edition
ISBN: 9780321967466
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 13, Problem 13.63CQ
Interpretation Introduction
Interpretation: IUPAC names and condensed structure formula
Conceptual Introduction: Dehydration reaction is a reaction in which loss of water molecule occur. Oxidation is defined as addition of oxygen, removal of hydrogen and removal of electron from that compound.
Addition of oxygen
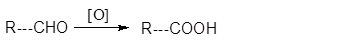
Removal of hydrogen
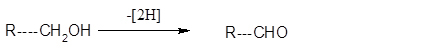
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
PLEASE ANSWER THE QUESTION!!!
3. SYNTHESIS. Propose a sequence of synthetic steps (FGI) that convert the starting
material (SM) into the Target molecule. For each FGI in your proposed synthesis,
specify the reagents / conditions, and draw the product(s) of that FGI. DO NOT
INCLUDE the FGI mxn in the answer you submit. If an FGI requires two reagent
sets, specify the order in which the reagent sets are added, e.g., i) Hg(OAc)2 / H₂O;
ii) NaBH4/MeOH. Indicate the stereochemistry (if any) of the products of each FGI.
FGI 1.
Me
Starting Material
Source of all carbons
in the Target molecule
(can use multiple copies)
Me
Me
Target molecule
+ enantiomer
curved arrows are used to illustate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction mechanism steps
Chapter 13 Solutions
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
Ch. 13.1 - Prob. 13.1QAPCh. 13.1 - Prob. 13.2QAPCh. 13.1 - Prob. 13.3QAPCh. 13.1 - Prob. 13.4QAPCh. 13.2 - Prob. 13.5QAPCh. 13.2 - Prob. 13.6QAPCh. 13.2 - Prob. 13.7QAPCh. 13.2 - Prob. 13.8QAPCh. 13.3 - Prob. 13.9QAPCh. 13.3 - Prob. 13.10QAP
Ch. 13.3 - Prob. 13.11QAPCh. 13.3 - Prob. 13.12QAPCh. 13.3 - Prob. 13.13QAPCh. 13.3 - Prob. 13.14QAPCh. 13.3 - Prob. 13.15QAPCh. 13.3 - Prob. 13.16QAPCh. 13.4 - Prob. 13.17QAPCh. 13.4 - Prob. 13.18QAPCh. 13.4 - Prob. 13.19QAPCh. 13.4 - Prob. 13.20QAPCh. 13.4 - Prob. 13.21QAPCh. 13.4 - Prob. 13.22QAPCh. 13.4 - Prob. 13.23QAPCh. 13.4 - Prob. 13.24QAPCh. 13.4 - Prob. 13.25QAPCh. 13.4 - Prob. 13.26QAPCh. 13.4 - a. Which of the functional groups alkene alcohol,...Ch. 13.4 - Prob. 13.28QAPCh. 13 - Prob. 13.29UTCCh. 13 - 13.30 Identify each of the following as an...Ch. 13 - Prob. 13.31UTCCh. 13 - Prob. 13.32UTCCh. 13 - Prob. 13.33UTCCh. 13 - Prob. 13.34UTCCh. 13 - Prob. 13.35UTCCh. 13 - Prob. 13.36UTCCh. 13 - Prob. 13.37AQAPCh. 13 - Prob. 13.38AQAPCh. 13 - Prob. 13.39AQAPCh. 13 - Prob. 13.40AQAPCh. 13 - Prob. 13.41AQAPCh. 13 - Prob. 13.42AQAPCh. 13 - Prob. 13.43AQAPCh. 13 - Prob. 13.44AQAPCh. 13 - Prob. 13.45AQAPCh. 13 - Prob. 13.46AQAPCh. 13 - Prob. 13.47AQAPCh. 13 - Prob. 13.48AQAPCh. 13 - Explain why each of the following compounds would...Ch. 13 - Prob. 13.50AQAPCh. 13 - Prob. 13.51AQAPCh. 13 - Prob. 13.52AQAPCh. 13 - Prob. 13.53AQAPCh. 13 - Prob. 13.54AQAPCh. 13 - Prob. 13.55AQAPCh. 13 - Dimethyl ether and ethyl alcohol both have the...Ch. 13 - Prob. 13.57AQAPCh. 13 - Draw the condensed or line-angle structural...Ch. 13 - Prob. 13.59AQAPCh. 13 - Menthol, which has a minty flavor, is used in...Ch. 13 - Prob. 13.61CQCh. 13 - Prob. 13.62CQCh. 13 - Prob. 13.63CQCh. 13 - Prob. 13.64CQCh. 13 - Prob. 13.65CQCh. 13 - Prob. 13.66CQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- At 90ºC the vapor pressure of ortho-xylene is 20 kPa and that of meta-xylene is 18 kPa. What is the composition of the vapor in equilibrium with a mixture in which the mole fraction of o-xylene is 0.60?arrow_forwardDraw the products of this reduction of a ketone with sodium borohydride. Use a dash or wedge bond to indicate the stereochemistry of substituents on asymmetric centers, where applicableIgnore any inorganic byproducts. 1) NaBH4 2) HCI/H2O Select to Drawarrow_forwardWhy do you think people who live at high altitudes are advised to add salt to water when boiling food like pasta? What mole fraction of NaCl is needed to raise the boiling point of H2O by 3˚C? Does the amount of salt added to water (typically about one teaspoon to four quarts of water) substantially change the boiling point? (Kb (H2O) = 0.51˚C/molal.)arrow_forward
- pls help asaparrow_forwardpls help asaparrow_forward9. Consider the following galvanic cell: Fe (s) | Fe(NO3)2 (aq) || Sn(NO3)2 (aq) | Sn (s) a. Write an equation for the half reactions occurring at the anode and cathode. b. Calculate the standard cell potential Show all of your work. c. Draw and label the galvanic cell, including the anode and cathode, direction of electron flow, and direction of ion migration.arrow_forward
- pls help asaparrow_forward11. Use the equation below to answer the following questions: 2 Al(s) + 3 Cd(NO3)2 (aq) → 2 Al(NO3)3 (aq) + 3 Cd(s) a. What is the net ionic equation for the reaction? b. Which species is a spectator ion in this reaction? Define a spectator ion. c. Identify the oxidizing agent and the reducing agent.arrow_forwardpls help asaparrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
ENVIRONMENTAL POLLUTION; Author: 7activestudio;https://www.youtube.com/watch?v=oxtMFmDTv3Q;License: Standard YouTube License, CC-BY