
Concept explainers
What major IR absorptions are present above
a. 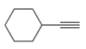 b.
b. 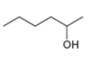 c.
c. 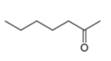 d.
d. 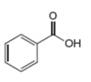
(a)
Interpretation: The major IR absorptions present above
Concept introduction: IR spectroscopy is used to identify the functional group present in a compound. Each and every bond vibrates at a characteristic frequency.
Answer to Problem 13.42P
The major IR absorption peaks are observed for
Explanation of Solution
The given compound is ethynylcyclohexane as shown below.
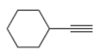
Figure 1
It contains
The major IR absorption peaks are observed for
(b)
Interpretation: The major IR absorptions present above
Concept introduction: IR spectroscopy is used to identify the functional group present in a compound. Each and every bond vibrates at a characteristic frequency.
Answer to Problem 13.42P
The major IR absorptions are observed for
Explanation of Solution
The given compound is hexan-2-ol as shown below.
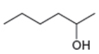
Figure 2
It contains
The major IR absorptions are observed for
(c)
Interpretation: The major IR absorptions present above
Concept introduction: IR spectroscopy is used to identify the functional group present in a compound. Each and every bond vibrates at a characteristic frequency.
Answer to Problem 13.42P
The major IR absorptions are observed for
Explanation of Solution
The given compound is hept-2-one as shown below.
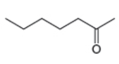
Figure 3
It contains
The major IR absorptions are observed for
(d)
Interpretation: The major IR absorptions are present above
Concept introduction: IR spectroscopy is used to identify the functional group present in a compound. Each and every bond vibrates at a characteristic frequency.
Answer to Problem 13.42P
The major IR absorptions are observed for
Explanation of Solution
The given compound is benzoic acid as shown below.
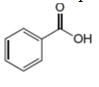
Figure 4
It contains,
The major IR absorptions are observed for
Want to see more full solutions like this?
Chapter 13 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
Additional Science Textbook Solutions
Campbell Biology: Concepts & Connections (9th Edition)
General, Organic, and Biological Chemistry - 4th edition
Genetic Analysis: An Integrated Approach (3rd Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Microbiology with Diseases by Body System (5th Edition)
- What are the major products of the following reaction? Please provide a detailed explanation and a drawing to show how the reaction proceeds.arrow_forwardWhat are the major products of the following enolate alkylation reaction? Please include a detailed explanation as well as a drawing as to how the reaction proceeds.arrow_forwardA block of zinc has an initial temperature of 94.2 degrees celcius and is immererd in 105 g of water at 21.90 degrees celcius. At thermal equilibrium, the final temperature is 25.20 degrees celcius. What is the mass of the zinc block? Cs(Zn) = 0.390 J/gxdegrees celcius Cs(H2O) = 4.18 J/gx degrees celcusarrow_forward
- Potential Energy (kJ) 1. Consider these three reactions as the elementary steps in the mechanism for a chemical reaction. AH = -950 kJ AH = 575 kJ (i) Cl₂ (g) + Pt (s) 2C1 (g) + Pt (s) Ea = 1550 kJ (ii) Cl (g)+ CO (g) + Pt (s) → CICO (g) + Pt (s) (iii) Cl (g) + CICO (g) → Cl₂CO (g) Ea = 2240 kJ Ea = 2350 kJ AH = -825 kJ 2600 2400 2200 2000 1800 1600 1400 1200 1000 a. Draw the potential energy diagram for the reaction. Label the data points for clarity. The potential energy of the reactants is 600 kJ 800 600 400 200 0 -200- -400 -600- -800- Reaction Progressarrow_forwardCan u help me figure out the reaction mechanisms for these, idk where to even startarrow_forwardHi, I need your help with the drawing, please. I have attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forward
- Hi, I need your help i dont know which one to draw please. I’ve attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forward5. Write the formation reaction of the following complex compounds from the following reactants: 6. AgNO₃ + K₂CrO₂ + NH₄OH → 7. HgNO₃ + excess KI → 8. Al(NO₃)₃ + excess NaOH →arrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. CO₂C2H5 + CH3-NH-NH,arrow_forward
- Draw the major product of this reaction N-(cyclohex-1-en-1-yl)-1-(pyrrolidino) reacts with CH2=CHCHO, heat, H3O+arrow_forwardDraw the starting material that would be needed to make this product through an intramolecular Dieckmann reactionarrow_forwardDraw the major product of this reaction. Nitropropane reacts + pent-3-en-2-one reacts with NaOCH2CH3, CH3CHOHarrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


