
Concept explainers
What are the major IR absorptions in the
a.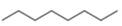 d.
d.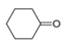
b.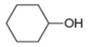
c. 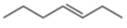 e.
e. 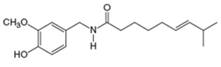
capsaicin
(spicy component of hot peppers)
(a)
Interpretation: The major IR absorptions in the functional group region for the given compound are to be predicted.
Concept introduction: IR spectroscopy is used to identify the functional group present in a compound. Each and every bond vibrates at a characteristic frequency.
Answer to Problem 13.19P
The major IR absorption peaks are observed for
Explanation of Solution
The given compound is octane. It contains
The major IR absorption peaks are observed for
(b)
Interpretation: The major IR absorptions in the functional group region for the given compound are to be predicted.
Concept introduction: IR spectroscopy is used to identify the functional group present in a compound. Each and every bond vibrates at a characteristic frequency.
Answer to Problem 13.19P
The major IR absorptions are observed for
Explanation of Solution
The given compound is cyclohexanol. It contains
The major IR absorptions are observed for
(c)
Interpretation: The major IR absorptions in the functional group region for the given compound are to be predicted.
Concept introduction: IR spectroscopy is used to identify the functional group present in a compound. Each and every bond vibrates at a characteristic frequency.
Answer to Problem 13.19P
The major IR absorptions are observed for
Explanation of Solution
The given compound is hept-3-ene. It contains
The major IR absorptions are observed for
(d)
Interpretation: The major IR absorptions in the functional group region for the given compound are to be predicted.
Concept introduction: IR spectroscopy is used to identify the functional group present in a compound. Each and every bond vibrates at a characteristic frequency.
Answer to Problem 13.19P
The major IR absorptions are observed for
Explanation of Solution
The given compound is cyclohexanone. It contains
The major IR absorptions are observed for
(e)
Interpretation: The major IR absorptions in the functional group region for the given compound are to be predicted.
Concept introduction: IR spectroscopy is used to identify the functional group present in a compound. Each and every bond vibrates at a characteristic frequency
Answer to Problem 13.19P
The major IR absorptions are observed for
Explanation of Solution
The given compound is capsaicin as shown below.
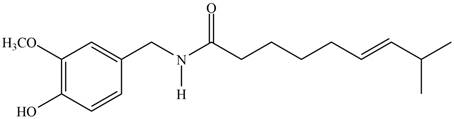
Figure 1
It contains
The major IR absorptions are observed for
Want to see more full solutions like this?
Chapter 13 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
Additional Science Textbook Solutions
Chemistry: Structure and Properties (2nd Edition)
General, Organic, and Biological Chemistry - 4th edition
The Cosmic Perspective (8th Edition)
Organic Chemistry (8th Edition)
- What is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forwardComplete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forward
- Q Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forwardb. ὋΗ CH3CH2OH H2SO4arrow_forward
- For the reaction A (g) → 3 B (g), Kp = 0.379 at 298 K. What is the value of ∆G for this reaction at 298 K when the partial pressures of A and B are 5.70 atm and 0.250 atm?arrow_forward14. Calculate the concentrations of Ag+, Ag(S2O3), and Ag(S2O3)23- in a solution prepared by mixing 150.0 mL of 1.00×10-3 M AgNO3 with 200.0 mL of 5.00 M Na2S2O3 Ag+ + S20 Ag(S203)¯ K₁ = 7.4 × 108 Ag(S203)¯ + S20¯ = Ag(S203) K₂ = 3.9 x 104arrow_forwardΗΝ, cyclohexanone pH 4-5 Draw Enamine I I CH3CH2Br THF, reflux H3O+ I Drawing Draw Iminium Ionarrow_forward
- :0: :0: Select to Add Arrows :0: (CH3)2NH :0: ■ Select to Add Arrows :0: :0: (CH3)2NH ■ Select to Add Arrowsarrow_forwardDraw the product of the following H action sequence. Ignore any inorganic byproducts formed. 1. (CH3CH2)2CuLi, THF 2. CH3Br Q Atoms, Bonds and Rings H Charges ㅁarrow_forwardPlease help me with this the problem is so confusingarrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

