
What products would result from the following processes?
Write an equation for each reaction.
a.
b.
c.
d.
e.
(a)
Interpretation:
The product formed when
Concept introduction:
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Answer to Problem 13.28E
No product is formed when
Explanation of Solution
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
The controlled oxidation of
No product is formed when
(b)
Interpretation:
The product formed when
Concept introduction:
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Answer to Problem 13.28E
The product formed when
![]()
Explanation of Solution
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Alcohols on heating at temperature
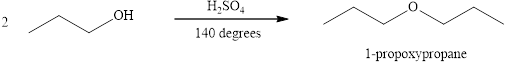
Figure 1
The product formed when
(c)
Interpretation:
The product formed when
Concept introduction:
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Answer to Problem 13.28E
The product formed when
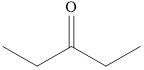
Explanation of Solution
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
As
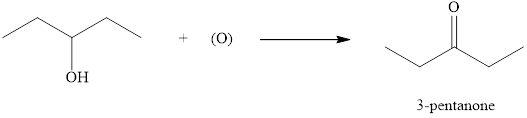
Figure 2
The product formed when
(d)
Interpretation:
The product formed when
Concept introduction:
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Answer to Problem 13.28E
The product formed when
![]()
Explanation of Solution
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Alcohols on heating at temperature
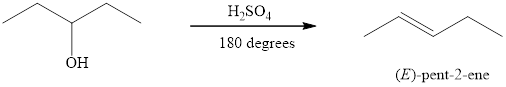
Figure 3
The product formed when
(e)
Interpretation:
The product formed when
Concept introduction:
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Answer to Problem 13.28E
The product formed when
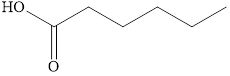
Explanation of Solution
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Generally, primary alcohols on oxidation give aldehyde. However, in the presence of excess oxidizing agent, aldehyde further oxidizes to carboxylic acid. Therefore, the product formed when

Figure 4
The product formed when
Want to see more full solutions like this?
Chapter 13 Solutions
Chemistry For Today: General, Organic, And Biochemistry, Loose-leaf Version
- Q10: (a) Propose a synthesis of C from A. (b) Propose a synthesis of C from B. Br Br ...\SCH 3 A B Carrow_forward9: Complete the missing entities for following reactions (e.g., major product(s), reactants, and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for reactions a) to d).arrow_forwardComplete the missing entities for following reactions (e.g., major product(s), reactants, and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for reactions a) to d).arrow_forward
- QUESTION 3: Provide the synthetic steps that convert the starting material into the product (no mechanism required). HO OH NH CH3 multiple steps 요요 H3Carrow_forwardQ6: Predict the effect of the changes given on the rate of the reaction below. CH3OH CH3Cl + NaOCH3 → CH3OCH3 + NaCl a) Change the substrate from CH3CI to CH31: b) Change the nucleophile from NaOCH 3 to NaSCH3: c) Change the substrate from CH3CI to (CH3)2CHCI: d) Change the solvent from CH3OH to DMSO.arrow_forwardQ3: Arrange each group of compounds from fastest SN2 reaction rate to slowest SN2 reaction rate. a) CI Cl فيكم H3C-Cl A B C D Br Br b) A B C Br H3C-Br Darrow_forward
- Q2: Group these solvents into either protic solvents or aprotic solvents. Acetonitrile (CH3CN), H₂O, Acetic acid (CH3COOH), Acetone (CH3COCH3), CH3CH2OH, DMSO (CH3SOCH3), DMF (HCON(CH3)2), CH3OHarrow_forwardSuppose the rate of evaporation in a hot, dry region is 1.76 meters per year, and the seawater there has a salinity of 35 ‰. Assuming a 93% yield, how much salt (NaCl) can be harvested each year from 1 km2 of solar evaporation ponds that use this seawater as a source?arrow_forwardhelparrow_forward
- Explain why only the lone pairs on the central atom are taken into consideration when predicting molecular shapearrow_forward(ME EX1) Prblm #9/10 Can you explain in detail (step by step) I'm so confused with these problems. For turmber 13 can u turn them into lewis dot structures so I can better understand because, and then as well explain the resonance structure part. Thanks for the help.arrow_forwardProblems 19 and 20: (ME EX1) Can you please explain the following in detail? I'm having trouble understanding them. Both problems are difficult for me to explain in detail, so please include the drawings and answers.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





