
Concept explainers
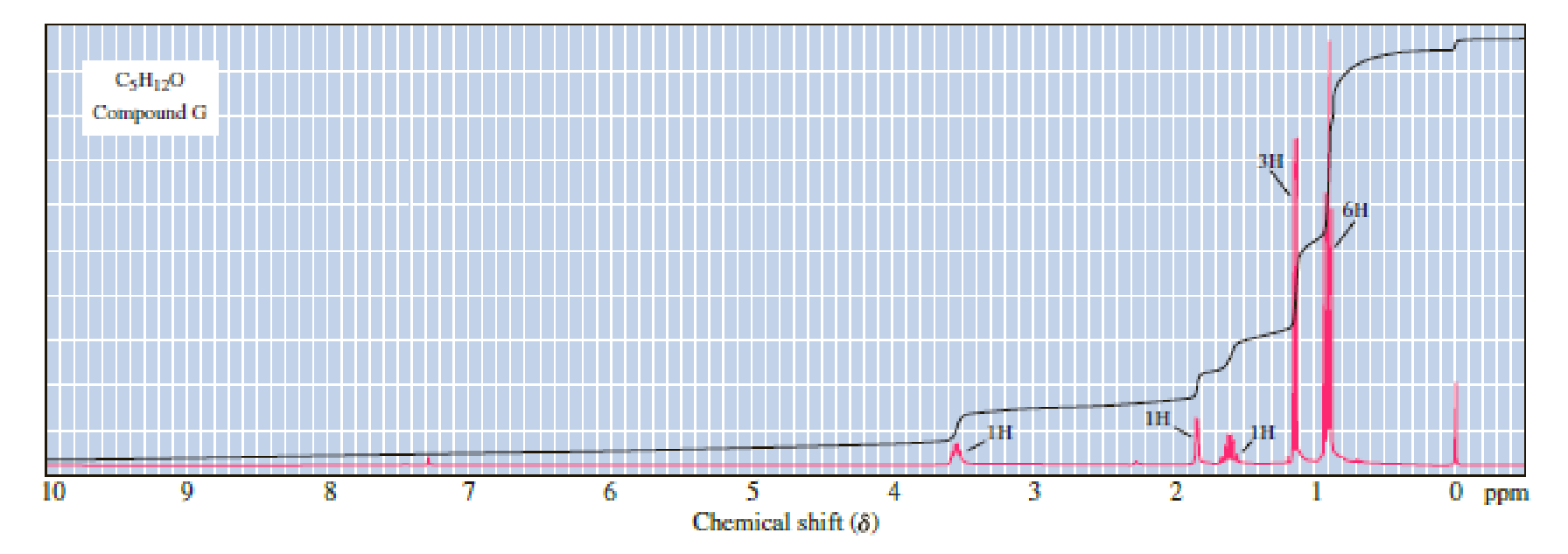
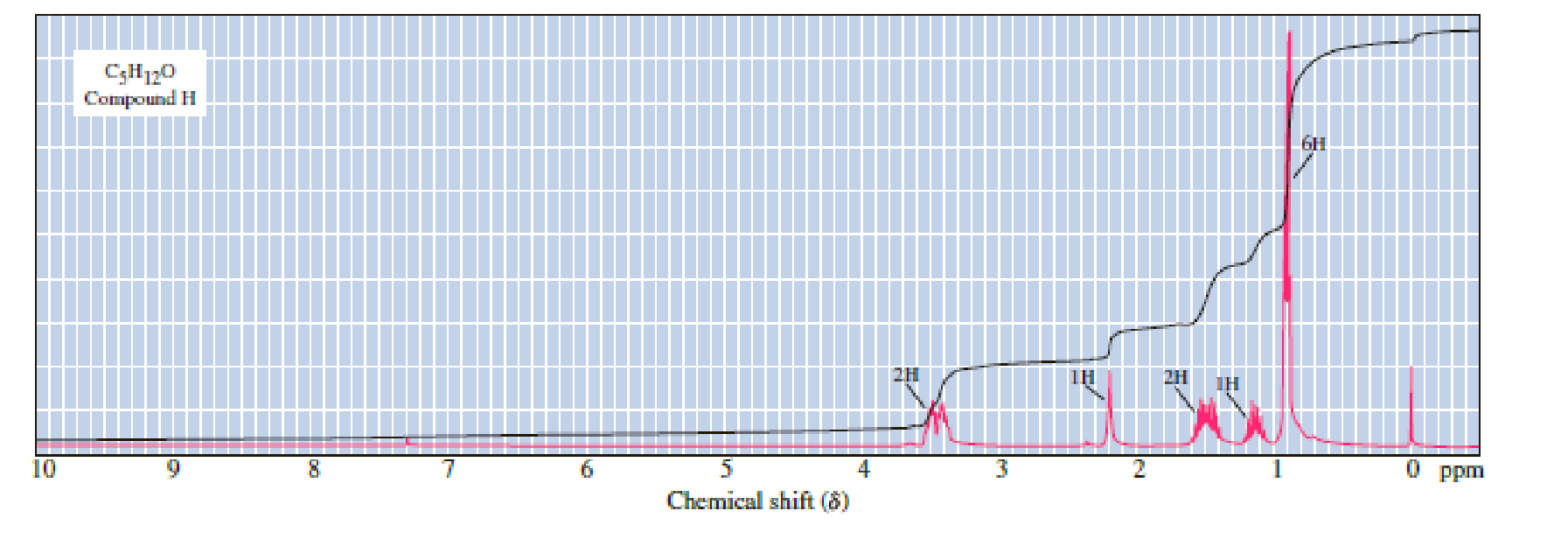
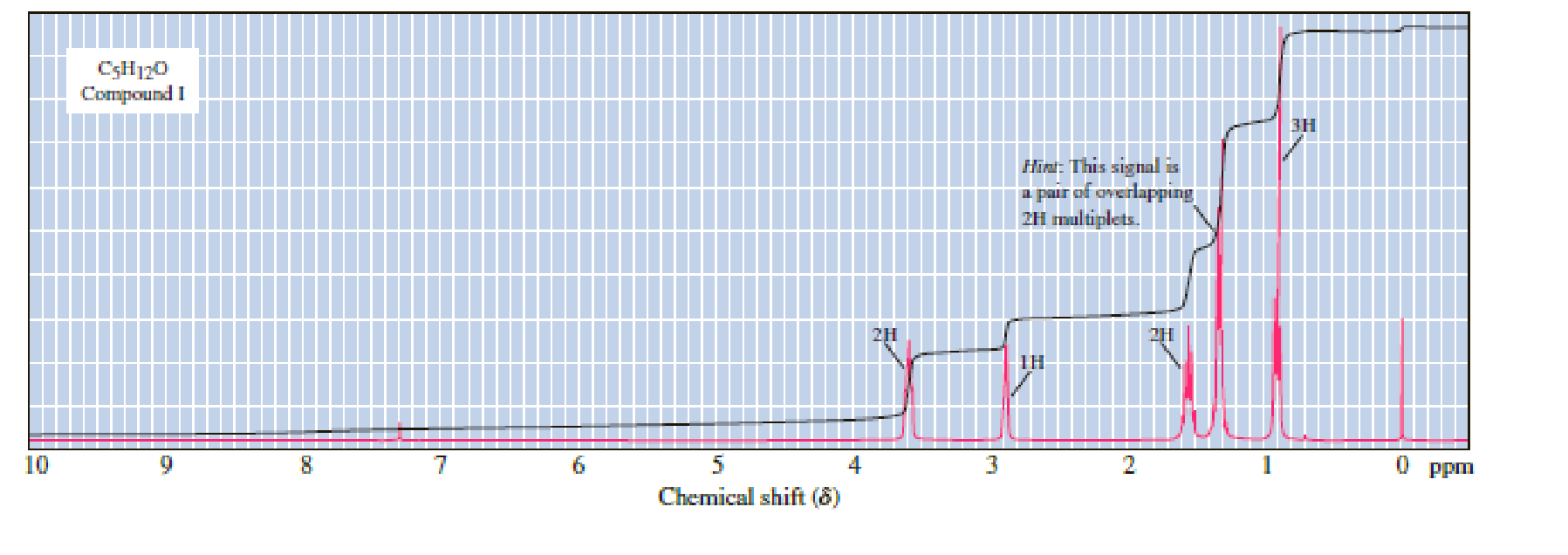
Following are 1H-NMR spectra for compounds G, H, and I, each with the molecular formula C5H12O. Each is a liquid at room temperature, is slightly soluble in water, and reacts with sodium metal with the evolution of a gas.
(a) Propose structural formulas of compounds G, H, and I.
(b) Explain why there are four lines between δ 0.86 and 0.90 for compound G.
(c) Explain why the 2H multiplets at δ 1.5 and 3.5 for compound H are so complex.
(a)
Interpretation:
Following compounds G,H and I stractural formula has to be proposed with the help of given molecular formula
Concept introduction:
The
Chemical shift: The NMR spectrum of any compound is taken with reference to a standard compound called reference compound. Generally, tetramethylsilane (TMS) is taken as the reference compound. The methyl protons of TMS are equivalent and produces only one sharp peak at the rightmost end of the scale.
The distance between the TMS signal and the signals produced by the compound is called the chemical shift. Chemical shift basically measures the shift in the signal position of the compound with respect to the reference signal.
Chemical shift in delta scale is given as,
Explanation of Solution
Index of Hydrogen Deficiency (IHD) calculation,
Given molecular formula is
We calculate the
From the molecular formula, there is an index of hydrogen deficiency is zero for these molecules, so there are no rings (or) double bonds.
The fact that the compounds are slightly soluble in water and react with sodium metal indicates that each molecule has an hydroxyl
The
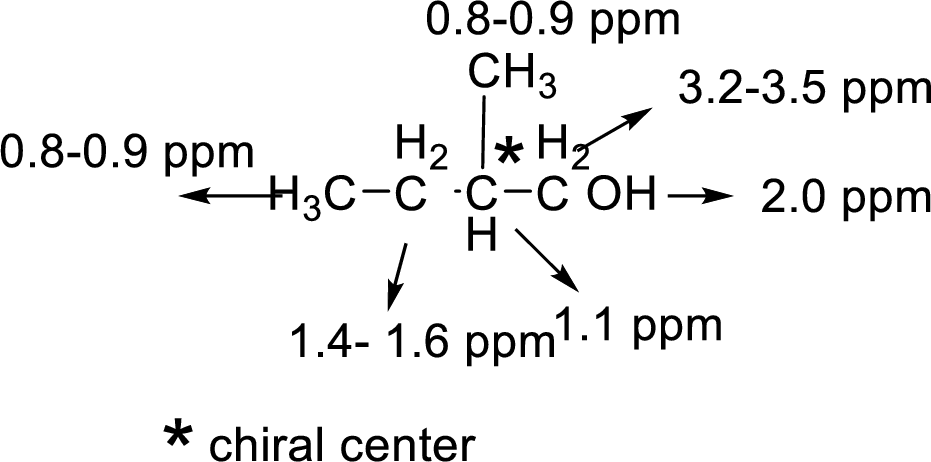
There are 5 peaks observed in the given
A multiplet is observed for 1 hydrogen at around
The one doublet is observed for 1 hydrogen at around
Another one multiplet is observed for one hydrogen at around
One doublet is observed for 3 hydrogens at around
The one doublet is observed for 6 hydrogens at around
Based on the above
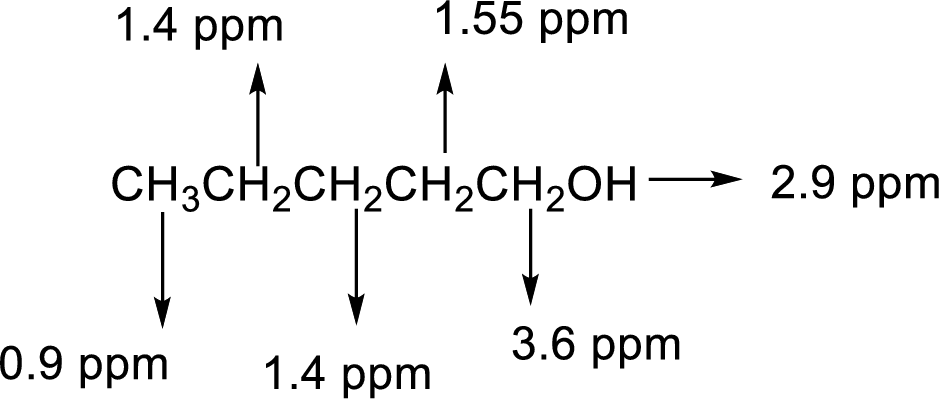
The
There are 5 peaks observed in the given
The
There are 5 peaks observed in the given
Based on the above
(b)
Interpretation:
The following compound-G has four lines observed in the range of
Concept introduction:
The
Diastereotopic: If the protons are not interchangeable by either of the symmetry operations, then the protons are Diastereotopic; the protons are not chemically equivalent if a chiral center present in the molecule.
Explanation of Solution
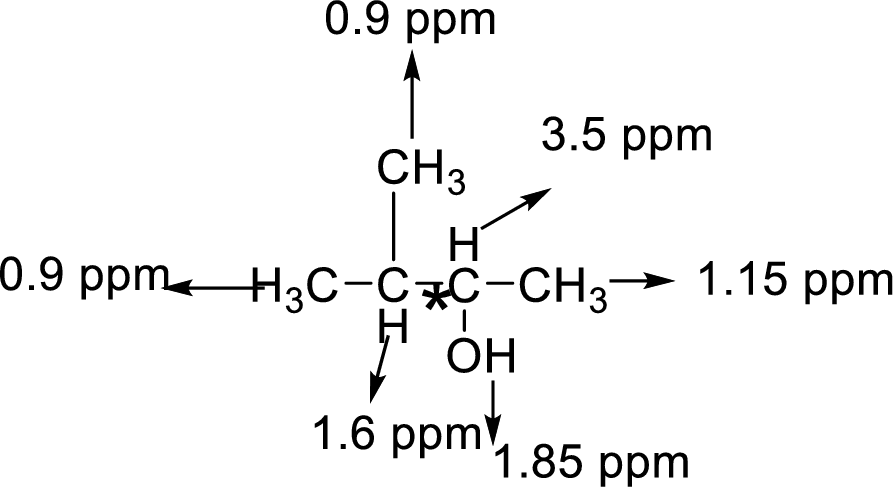
Let us consider the given compound-G:
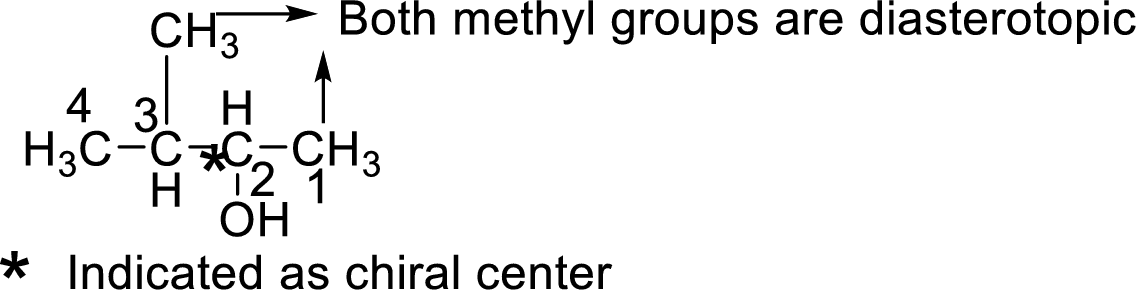
Above the molecule, the carbon atom 2 attached with hydroxyl
That is makes the two methyl groups are diastereotopic, so they have different chemical shifts, the four lines are actually two doublets.
(c)
Interpretation:
The Following compound-H is two hydrogens appread at multiplet, this compound is complex or not, the behaind reason should be explained.
Concept introduction:
The
Chemical shift: The NMR spectrum of any compound is taken with reference to a standard compound called reference compound. Generally, tetramethylsilane (TMS) is taken as the reference compound. The methyl protons of TMS are equivalent and produces only one sharp peak at the rightmost end of the scale.
The distance between the TMS signal and the signals produced by the compound is called the chemical shift. Chemical shift basically measures the shift in the signal position of the compound with respect to the reference signal.
Chemical shift in delta scale is given as,
Diastereotopic: If the protons are not interchangeable by either of the symmetry operations, then the protons are Diastereotopic; the protons are not chemically equivalent if a chiral center present in the molecule.
Explanation of Solution
Let us consider the given compound-H:
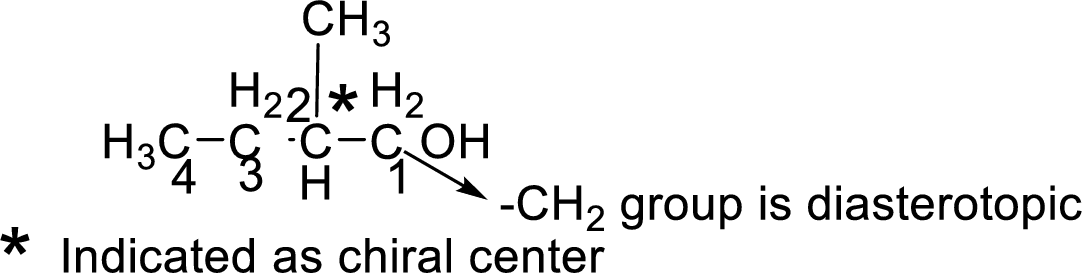
Above the molecule, the carbon atom 2 attached with hydroxyl
This chiral center makes the adjacent
Want to see more full solutions like this?
Chapter 13 Solutions
Organic Chemistry, Loose-leaf Version
Additional Science Textbook Solutions
Chemistry: Structure and Properties (2nd Edition)
Physical Universe
Physical Science
Essentials of Human Anatomy & Physiology (12th Edition)
Fundamentals Of Thermodynamics
- 2) Draw the correct chemical structure (using line-angle drawings / "line structures") from their given IUPAC name: a. (E)-1-chloro-3,4,5-trimethylhex-2-ene b. (Z)-4,5,7-trimethyloct-4-en-2-ol C. (2E,6Z)-4-methylocta-2,6-dienearrow_forwardපිපිම Draw curved arrows to represent the flow of electrons in the reaction on the left Label the reactants on the left as either "Acid" or "Base" (iii) Decide which direction the equilibrium arrows will point in each reaction, based on the given pk, values (a) + H-O H 3-H + (c) H" H + H****H 000 44-00 NH₂ (e) i Дон OH Ө NHarrow_forward3) Label the configuration in each of the following alkenes as E, Z, or N/A (for non-stereogenic centers). 00 E 000 N/A E Br N/A N/A (g) E N/A OH E (b) Oz N/A Br (d) 00 E Z N/A E (f) Oz N/A E (h) Z N/Aarrow_forward
- 6) Fill in the missing Acid, pKa value, or conjugate base in the table below: Acid HCI Approximate pK, -7 Conjugate Base H-C: Hydronium (H₂O') -1.75 H-O-H Carboxylic Acids (RCOOH) Ammonium (NH4) 9.24 Water (H₂O) H-O-H Alcohols (ROH) RO-H Alkynes R--H Amines 25 25 38 HOarrow_forward5) Rank the following sets of compounds in order of decreasing acidity (most acidic to least acidic), and choose the justification(s) for each ranking. (a) OH V SH я вон CH most acidic (lowst pKa) least acidic (highest pKa) Effect(s) Effect(s) Effect(s) inductive effect O inductive effect O inductive effect electronegativity electronegativity O electronegativity resonance polarizability resonance polarizability O resonance O polarizability hybridization Ohybridization O hybridization оarrow_forwardHow negatively charged organic bases are formed.arrow_forward
- Nonearrow_forward1) For the following molecules: (i) Label the indicated alkenes as either cis (Z), trans (E), or N/A (for non-stereogenic centers) by bubbling in the appropriate label on the molecule. (ii) Complete the IUPAC name located below the structure (HINT: Put the letter of the configuration in parentheses at the beginning of the name!) E z N/A ()-3,4,6-trimethylhept-2-ene E Oz O N/A ()-3-ethyl-1-fluoro-4-methylhex-3-ene E -+- N/A Me )-2,3-dimethylpent-2-ene (d) (b) E O N/A Br ()-5-bromo-1-chloro-3-ethyloct-4-ene ОЕ Z N/A Et (___)-3-ethyl-4-methylhex-3-ene E (f) Oz N/A z N/A HO (4.7)-4-(2-hydroxyethyl)-7-methylnona-4,7-dien-2-onearrow_forwardO 9:21AM Tue Mar 4 ## 64% Problem 51 of 15 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. H :0: CI. AI :CI: :CI: Cl AI Select to Add Arrows Select to Add Arrows O: Cl :CI: :0: H CI: CI CO Select to Add Arrows Select to Add Arrows :O: CI :0: Cl. 10: AIarrow_forward
- (i) Draw in the missing lone pair(s) of electrons of the reactants on the left (ii) Draw (curved) arrows to show the flow of electrons in the acid/base reaction on the left (iii) Draw the products of the acid/base on the right (iv) Select the correct label for each product as either "conjugate acid" or "conjugate base" (a) JOH OH NH₂ acid base (b) De "H conjugate acid conjugate acid conjugate base conjugate base acid base conjugate acid conjugate base conjugate acid conjugate base acid basearrow_forwardCould someone answer this NMR and explain please Comment on the general features of the 1H-NMR spectrum of isoamyl ester provided below.arrow_forwardMacmillan Learning Draw the acyl chloride that would give the ketone shown using the Friedel-Crafts acylation reaction. Select Draw Templates More с H о Cl 2Q Erase AICI₂arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
