
Organic Chemistry, Loose-leaf Version
8th Edition
ISBN: 9781305865549
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 13.15P
Following are three compounds with the molecular formula C4H8O2 and three 1H-NMR spectra. Assign each compound its correct spectrum and assign all signals to their corresponding hydrogens.
(1) 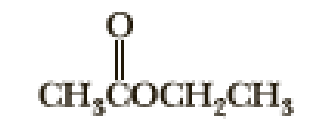
(2) 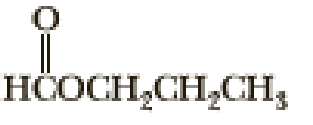
(3) 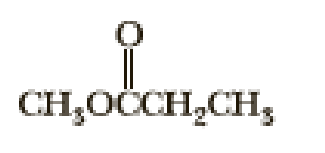
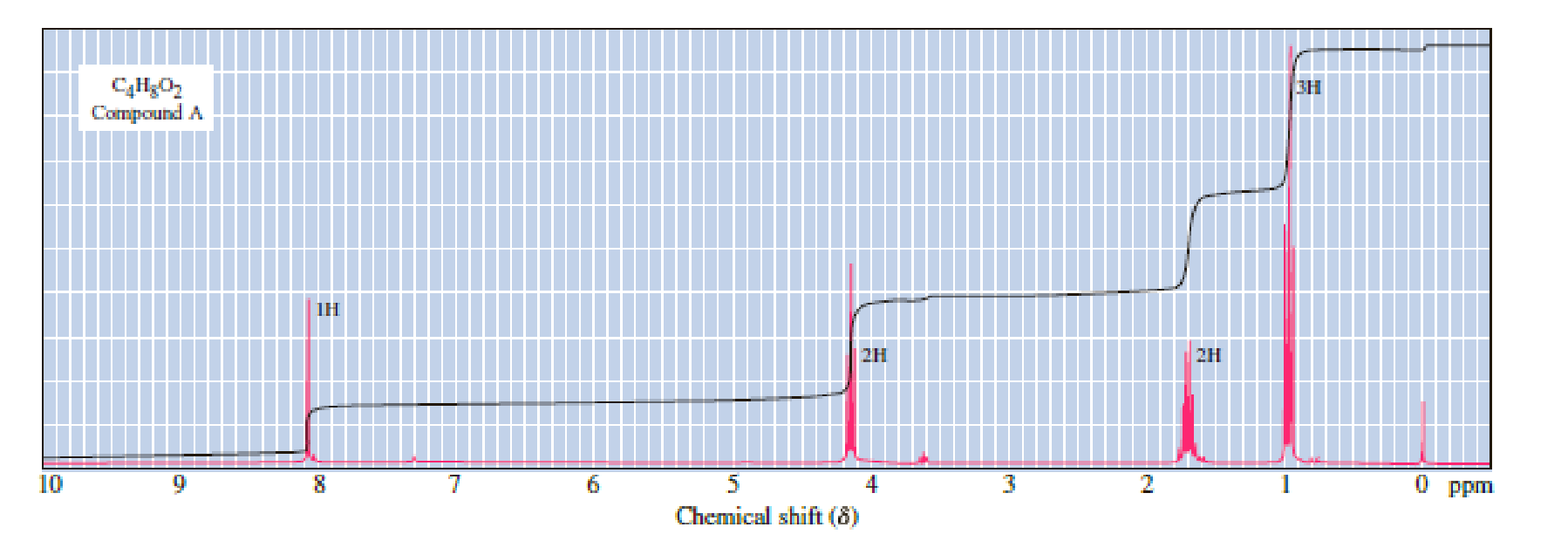
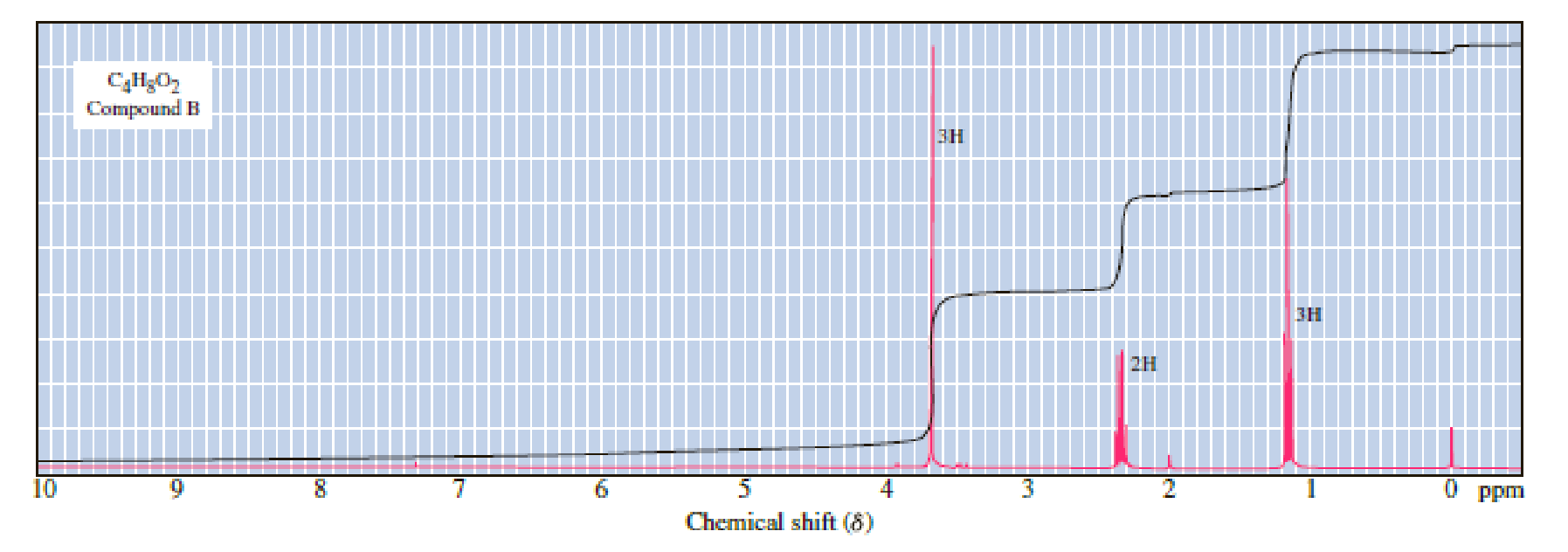
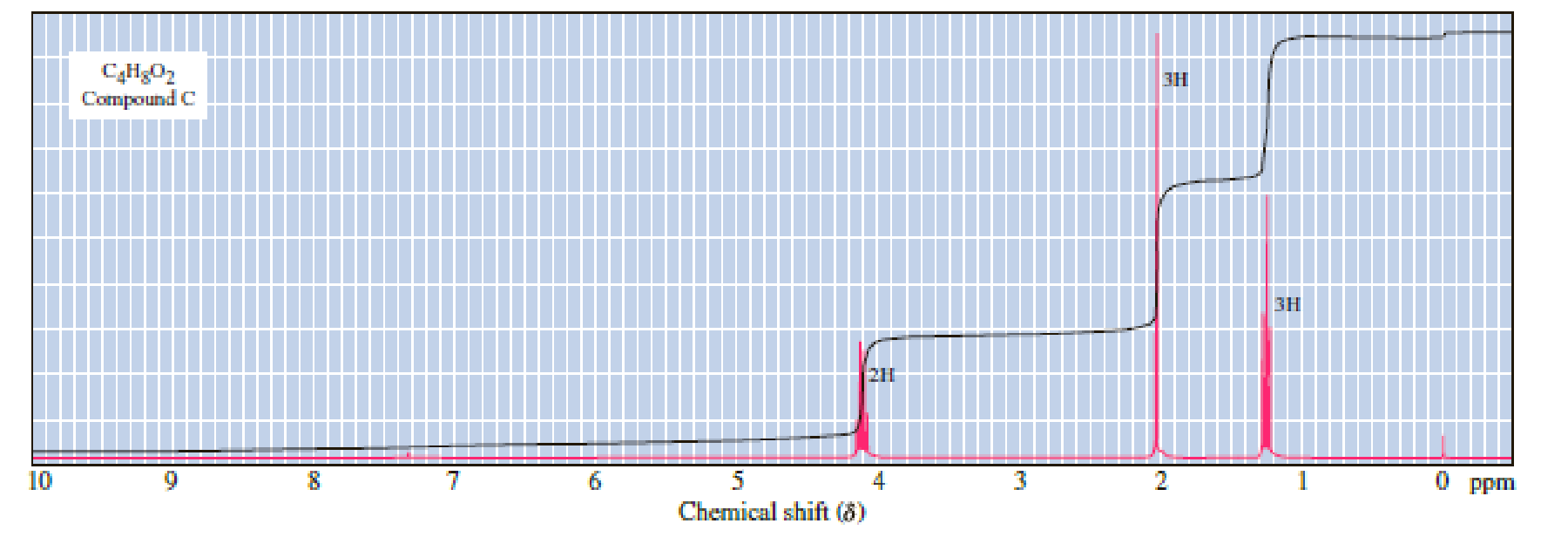
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
I need the most help figuring out how to find [I^-] mol/ L, [S2O8^2-] mol/L. 1st and 2nd Blank columns.
Can someone help me whats the issue?
a. The change in the Gibbs energy of a certain constant pressure process is found to fit the expression:
AG-85.1 J mol −1 +36.5 J mol ¹K-1 × T
A. Calculate the value of AS for the process.
B. Next, use the Gibbs-Helmholtz equation:
(a(AG/T))
ΔΗ
-
T2
to calculate the value of AH for the process.
Chapter 13 Solutions
Organic Chemistry, Loose-leaf Version
Ch. 13.2 - Calculate the ratio of nuclei in the higher spin...Ch. 13.5 - State the number of sets of equivalent hydrogens...Ch. 13.5 - Each compound gives only one signal in its 1H-NMR...Ch. 13.6 - The line of integration of the two signals in the...Ch. 13.7 - Following are two constitutional isomers with the...Ch. 13.8 - Following are pairs of constitutional isomers....Ch. 13.10 - Following is a 1H-NMR spectrum of 2-butanol....Ch. 13.11 - Explain how to distinguish between the members of...Ch. 13 - Prob. 13.9PCh. 13 - Prob. 13.10P
Ch. 13 - Prob. 13.11PCh. 13 - Following are structural formulas for three...Ch. 13 - Following arc structural formulas for the cis...Ch. 13 - Prob. 13.14PCh. 13 - Following are three compounds with the molecular...Ch. 13 - Following are 1H-NMR spectra for compounds D, E,...Ch. 13 - Following are 1H-NMR spectra for compounds G, H,...Ch. 13 - Propose a structural formula for compound J,...Ch. 13 - Compound K, molecular formula C6H14O, readily...Ch. 13 - Compound M, molecular formula C5H10O, readily...Ch. 13 - Following is the 1H-NMR spectrum of compound O,...Ch. 13 - Treatment of compound P with BH3 followed by...Ch. 13 - The 1H-NMR spectrum of compound R, C6H14O,...Ch. 13 - Write structural formulas for the following...Ch. 13 - Prob. 13.25PCh. 13 - Ascaridole is a natural product that has been used...Ch. 13 - The 13C-NMR spectrum of 3-methyl-2-butanol shows...Ch. 13 - Prob. 13.28P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
2. Why is it that the range of resting blood pressures of humans is best represented by a bell-shaped curve co...
Human Biology: Concepts and Current Issues (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the structure of 1-bromo-2-fluoroethane. Part 1 of 2 Draw the Newman projection for the anti conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. ✡ ぬ Part 2 of 2 H H F Br H H ☑ Draw the Newman projection for the gauche conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. H F Br H Harrow_forwardPlease help me answer this question. I don't understand how or where the different reagents will attach and it's mostly due to the wedge bond because I haven't seen a problem like this before. Please provide a detailed explanation and a drawing showing how it can happen and what the final product will look like.arrow_forwardWhich of the following compounds is the most acidic in the gas phase? Group of answer choices H2O SiH4 HBr H2Sarrow_forward
- Which of the following is the most acidic transition metal cation? Group of answer choices Fe3+ Sc3+ Mn4+ Zn2+arrow_forwardBased on the thermodynamics of acetic acid dissociation discussed in Lecture 2-5, what can you conclude about the standard enthalpy change (ΔHo) of acid dissociation for HCl? Group of answer choices You cannot arrive at any of the other three conclusions It is a positive value It is more negative than −0.4 kJ/mol It equals −0.4 kJ/molarrow_forwardPLEASE HELP URGENT!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY