
Concept explainers
13.106 Hydrazine,
(a) Which reaction occurs at the anode and which at the cathode?
(b) What is the net cell reaction?
(C) If the cell is to produce 0.50 A of current for 50.0 h, what mass in grams of hydrazine must be present?
(d) What mass in grams of
(b)
Interpretation:
To identify the net reaction.
Concept introduction:
- The net reaction is the total reaction including all reactants and products.
- It is obtained via addition of the half reactions.
Answer to Problem 13.106PAE
Solution:
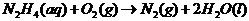
Explanation of Solution
In order to find out the reaction, we must include the reduction-oxidation reactions. They always take place together, since one donates electrons and the other receives them.
In this case, the oxidation reaction: hydrazine oxidation to nitrogen gas. The reduction is oxygen forming hydroxide ion via the addition of electron
Adding both reactions, getting rid of repeating/same units on the left/right side of the equation:
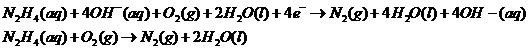
Therefore, the reaction taking place is:
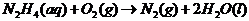
(c)
Interpretation:
To calculate the mass of hydrazine present in given amount of time and current.
Concept introduction:
- A stands for ampere, the amount of current.
- Time and current can be used to calculate total charge.
- The total charge relates mol of e- and Faraday constant.
Answer to Problem 13.106PAE
Solution:
Explanation of Solution
This consist of the following steps:
1st Step: Calculate the total amount of charge.
2nd Step: Calculate the total amount of moles of hydrazine
3rd Step: Calculate the mass of hydrazine
(d)
Interpretation:
To calculate the mass oxygen required
Concept introduction:
- Use stoichiometric ratios.
Answer to Problem 13.106PAE
Solution:
Explanation of Solution
Relate the moles of oxygen and hydrazine:
1st Step: Calculate total moles of oxygen required
The ratio is 1:1 since 1 mol of hydrazine requires 1 mol of Oxygen gas to react.
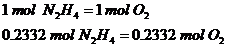
2nd Step: Calculate the total mass of oxygen gas
The mass is exactly the same as hydrazine since they have 1:1 ratio and similar molar masses.
Want to see more full solutions like this?
Chapter 13 Solutions
Chemistry for Engineering Students
- Hi can you please help me solve this problem? thank youarrow_forwardAn electrode process takes place at a metal-solution interface. Indicate the current condition that must be met for Faradaic rectification to occur.arrow_forwardAt a metal-solution interface, an electron is exchanged, and the symmetry factor beta < 0.5 is found in the Butler-Volmer equation. What does this indicate?arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





