
CHEMISTRY:ATOMS FIRST-2 YEAR CONNECT
2nd Edition
ISBN: 9781260592320
Author: Burdge
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 12.6, Problem 12.7WE
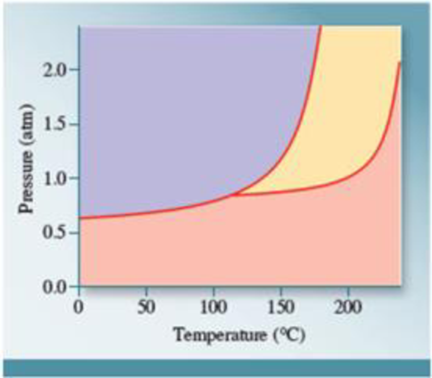
Using the following phase diagram, (a) determine the normal boiling point and the normal melting point of the substance, (b) determine the physical state of the substance at 2 atm and 110°C, and (c) determine the pressure and temperature that correspond to the triple point of the substance.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Select an amino acid that has and N-H or O-H bond in its R-group (you have 8 to choose from!). Draw at least two water molecules interacting with the R-group of the amino acid.
Is this aromatic?
CHEM2323
E
Tt
PS CH03
Draw and name all monobromo derivatives of pentane, C5H11Br.
Problem 3-33
Name:
Draw structures for the following:
(a) 2-Methylheptane
(d) 2,4,4-Trimethylheptane
Problem 3-35
(b) 4-Ethyl-2,2-dimethylhexane
(e) 3,3-Diethyl-2,5-dimethylnonane
(c) 4-Ethyl-3,4-dimethyloctane
2
(f) 4-Isopropyl-3-methylheptane
KNIE>
Chapter 12 Solutions
CHEMISTRY:ATOMS FIRST-2 YEAR CONNECT
Ch. 12.2 - Prob. 12.1WECh. 12.2 - Prob. 1PPACh. 12.2 - Prob. 1PPBCh. 12.2 - The diagram on the left depicts a system at room...Ch. 12.2 - Prob. 12.2.1SRCh. 12.2 - Prob. 12.2.2SRCh. 12.2 - Prob. 12.2.3SRCh. 12.2 - Prob. 12.2.4SRCh. 12.3 - Prob. 12.2WECh. 12.3 - When silver crystallizes, it forms face-centered...
Ch. 12.3 - The density of sodium metal is 0.971 g/cm3 and the...Ch. 12.3 - Prob. 12.3.1SRCh. 12.3 - Prob. 12.3.2SRCh. 12.4 - How many of each ion are contained within a unit...Ch. 12.4 - Referring to Figure 12.23, determine how many of...Ch. 12.4 - Referring to Figure 12.23, determine how many of...Ch. 12.4 - Prob. 3PPCCh. 12.4 - The edge length of the NaCl unit cell is 564 pm....Ch. 12.4 - Prob. 4PPACh. 12.4 - NiO also adopts the face-centered cubic...Ch. 12.4 - The metal iridium (Ir) crystallizes with a...Ch. 12.4 - Prob. 5PPACh. 12.4 - Copper crystallizes in a face-centered cubic...Ch. 12.4 - Given that the diameter and average mass of a...Ch. 12.5 - (a) Calculate the amount of heat deposited oil the...Ch. 12.5 - Calculate the amount of energy (in kilojoules)...Ch. 12.5 - Determine the final state and temperature of 100 g...Ch. 12.5 - Prob. 6PPCCh. 12.5 - Prob. 12.5.1SRCh. 12.5 - Prob. 12.5.2SRCh. 12.6 - Using the following phase diagram, (a) determine...Ch. 12.6 - Use the following phase diagram to (a) determine...Ch. 12.6 - Prob. 7PPBCh. 12.6 - Prob. 7PPCCh. 12.6 - Prob. 12.6.1SRCh. 12 - Explain why liquids, unlike gases, are virtually...Ch. 12 - What is surface tension? What is the relationship...Ch. 12 - Prob. 12.3QPCh. 12 - Prob. 12.4QPCh. 12 - Prob. 12.5QPCh. 12 - Prob. 12.6QPCh. 12 - Prob. 12.7QPCh. 12 - Why does the viscosity of a liquid decrease with...Ch. 12 - Why is ice less dense than water?Ch. 12 - Prob. 12.10QPCh. 12 - Prob. 12.11QPCh. 12 - Prob. 12.12QPCh. 12 - Prob. 12.13QPCh. 12 - Predict which of the following liquids has greater...Ch. 12 - Prob. 12.15QPCh. 12 - Vapor pressure measurements at several different...Ch. 12 - The vapor pressure of liquid X is lower than that...Ch. 12 - Prob. 12.18QPCh. 12 - Prob. 12.19QPCh. 12 - Define the following terms: crystalline solid,...Ch. 12 - Prob. 12.21QPCh. 12 - Classify the solid states in terms of crystal...Ch. 12 - Prob. 12.23QPCh. 12 - Define X-ray diffraction. What are the typical...Ch. 12 - Prob. 12.25QPCh. 12 - What is the coordination number of each sphere in...Ch. 12 - Calculate the number of spheres that would be...Ch. 12 - Prob. 12.28QPCh. 12 - Barium metal crystallizes in a body-centered cubic...Ch. 12 - Prob. 12.30QPCh. 12 - Europium crystallizes in a body-centered cubic...Ch. 12 - Crystalline silicon has a cubic structure. The...Ch. 12 - Prob. 12.33QPCh. 12 - Prob. 12.34QPCh. 12 - Prob. 12.35QPCh. 12 - Prob. 12.36QPCh. 12 - Shown here is a zinc oxide unit cell. What is the...Ch. 12 - Describe and give examples of the following types...Ch. 12 - Prob. 12.39QPCh. 12 - A solid is hard, brittle, and electrically...Ch. 12 - A solid is soft and has a low melting point (below...Ch. 12 - Prob. 12.42QPCh. 12 - Which of the following are molecular solids and...Ch. 12 - Prob. 12.44QPCh. 12 - Prob. 12.45QPCh. 12 - What is a phase change? Name all possible changes...Ch. 12 - What is the equilibrium vapor pressure of a...Ch. 12 - Use any one of the phase changes to explain what...Ch. 12 - Define the following terms: (a) molar heat of...Ch. 12 - Prob. 12.50QPCh. 12 - What can we learn about the intermolecular forces...Ch. 12 - The greater the molar heat of vaporization of a...Ch. 12 - Prob. 12.53QPCh. 12 - A closed container of liquid pentane (bp = 36.1C)...Ch. 12 - What is critical temperature? What is the...Ch. 12 - Prob. 12.56QPCh. 12 - How do the boiling points and melting points of...Ch. 12 - Prob. 12.58QPCh. 12 - Prob. 12.59QPCh. 12 - Prob. 12.60QPCh. 12 - Which of the following phase transitions gives off...Ch. 12 - Prob. 12.62QPCh. 12 - Prob. 12.63QPCh. 12 - Prob. 12.64QPCh. 12 - Prob. 12.65QPCh. 12 - Prob. 12.66QPCh. 12 - Prob. 12.67QPCh. 12 - How is the rate of evaporation of a liquid...Ch. 12 - Explain why steam at 100C causes more serious bums...Ch. 12 - The following compounds, listed with then- boiling...Ch. 12 - Prob. 12.71QPCh. 12 - Prob. 12.72QPCh. 12 - Explain how waters phase diagram differs from...Ch. 12 - The blades of ice skates are quite thin, so the...Ch. 12 - A length of wire is placed on top of a block of...Ch. 12 - Prob. 12.76QPCh. 12 - A phase diagram of water is shown. Label the...Ch. 12 - Prob. 12.78QPCh. 12 - Prob. 12.79QPCh. 12 - Prob. 12.80QPCh. 12 - Prob. 12.81QPCh. 12 - Prob. 12.82QPCh. 12 - The average distance between base pairs measured...Ch. 12 - A CO2 fire extinguisher is located on the outside...Ch. 12 - What is the vapor pressure of mercury at its...Ch. 12 - Prob. 12.86QPCh. 12 - The liquid-vapor boundary line in the phase...Ch. 12 - Prob. 12.88QPCh. 12 - Prob. 12.89QPCh. 12 - A student is given four solid samples labeled W,...Ch. 12 - Prob. 12.91QPCh. 12 - The diagram shows a kettle of boiling water....Ch. 12 - The south pole of Mars is covered with solid...Ch. 12 - The properties of gases, liquids, and solids...Ch. 12 - The standard enthalpy of formation of gaseous...Ch. 12 - Prob. 12.96QPCh. 12 - Under the same conditions of temperature and...Ch. 12 - The distance between Li+ and Cl is 257 pm in solid...Ch. 12 - Heat of hydration, that is, the heat change that...Ch. 12 - The fluorides of the second period elements and...Ch. 12 - Calculate the H for the following processes at...Ch. 12 - Prob. 12.102QPCh. 12 - Prob. 12.103QPCh. 12 - Ozone (O3) is a strong oxidizing agent that can...Ch. 12 - A sample of limestone (CaCO3) is heated in a...Ch. 12 - Carbon and silicon belong to Group 4A of the...Ch. 12 - Prob. 12.107QPCh. 12 - A 1.20-g sample of water is injected into an...Ch. 12 - What are the advantages of cooking the vegetable...Ch. 12 - A quantitative measure of how efficiently spheres...Ch. 12 - The phase diagram of helium is shown. Helium is...Ch. 12 - The phase diagram of sulfur is shown. (a) How many...Ch. 12 - Prob. 12.113QPCh. 12 - Argon crystallizes in the face-centered cubic...Ch. 12 - Given the phase diagram of carbon, answer the...Ch. 12 - Prob. 12.116QPCh. 12 - Swimming coaches sometimes suggest that a drop of...Ch. 12 - Prob. 12.118QPCh. 12 - Why do citrus growers spray their trees with water...Ch. 12 - Calcium metal crystallizes in a face-centered...Ch. 12 - A student heated a beaker of cold water (on a...Ch. 12 - The compound diclilorodifluoromethane (CCl2F2) has...Ch. 12 - Iron crystallizes in a body-centered cubic...Ch. 12 - Sketch the cooling curves of water from about 110C...Ch. 12 - Prob. 12.125QPCh. 12 - A sampleof water shows the following behavior as...Ch. 12 - A closed vessel of volume 9.6 L contains 2.0 g of...Ch. 12 - The electrical conductance of copper metal...Ch. 12 - Assuming ideal behavior, calculate the density of...Ch. 12 - Explain why drivers are advised to use motor oil...Ch. 12 - Prob. 12.131QPCh. 12 - Silicon used in computer chips must have an...Ch. 12 - Prob. 12.133QPCh. 12 - Prob. 12.1KSPCh. 12 - Prob. 12.2KSPCh. 12 - Prob. 12.3KSPCh. 12 - Prob. 12.4KSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Problem 3-42 Consider 2-methylbutane (isopentane). Sighting along the C2-C3 bond: (a) Draw a Newman projection of the most stable conformation. (b) Draw a Newman projection of the least stable conformation. Problem 3-44 Construct a qualitative potential-energy diagram for rotation about the C-C bond of 1,2-dibromoethane. Which conformation would you expect to be most stable? Label the anti and gauche conformations of 1,2- dibromoethane. Problem 3-45 Which conformation of 1,2-dibromoethane (Problem 3-44) would you expect to have the largest dipole moment? The observed dipole moment of 1,2-dibromoethane is µ = 1.0 D. What does this tell you about the actual conformation of the molecule?arrow_forwardGas Law Studies 1. Mass of zinc Determination of 0.899 2) Moles of zinc 0.01361 mol 3.) Moles of hydrogen 00? ← I was told to calculate this number from mole of zinc. 350m So does that mean it will be 0.01361 mol too? 4 Volume of water collected (mL) 5) VL of water collected (Liters) 0.350 L 6) Temp of water collected (°C) 7) Temp of water collected (°K) 8) Atmospheric pressure (mm) 9) Vapor pressure of water (mm) 10) Corrected pressure of hydrogen 20% 29°C 764.0mm Hg (mm) 17.5mm 11) Corrected pressure of hydrogen (atm) 12) Experimentally calculated value of 19 13. Literature value of R 14) % Error 15) Suggest reasons for the % error (#14)arrow_forwardNo wedge or dashes. Do proper structure. Provide steps and explanation.arrow_forward
- 10 Question (1 point) Draw curved arrow notation to indicate the proton transfer between NaOH and CH3CO₂H. 2nd attempt :0- H See Periodic Table See Hint Draw the products of the proton transfer reaction. Don't add a + sign between the products.arrow_forwardProvide steps and explanation please.arrow_forwardProvide steps to name and label for understanding.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning
Chemical Equilibria and Reaction Quotients; Author: Professor Dave Explains;https://www.youtube.com/watch?v=1GiZzCzmO5Q;License: Standard YouTube License, CC-BY