
Fundamentals of General, Organic, and Biological Chemistry, Books a la Carte Plus Mastering Chemistry with Pearson eText -- Access Card Package (8th Edition)
8th Edition
ISBN: 9780134261256
Author: John McMurray, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 12.4, Problem 12.5P
Draw the following three isomers of C5H12 as condensed structures:
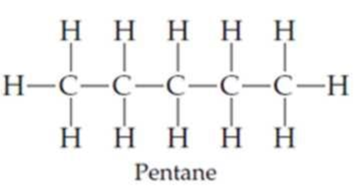
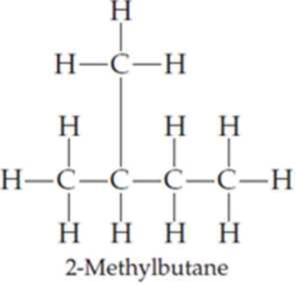
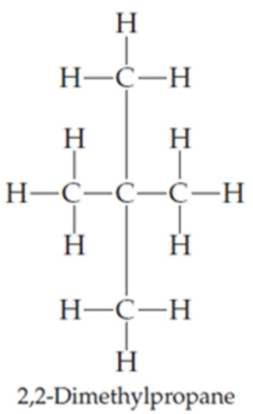
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Can koch's postulates of disease causation be applied to non-microorganism disease pathogens (such as molecules)?
Here is my literature Beta Carotene HPLC analysis graph.
Can you help me explain what each peak is at each retention time?
Thank You :D
I have a literature B-Carotene HPLC graph in which showcases a retention time of roughly 23.6 and 25.1.
Please help me compare my two different Anti-Oxidant Juice graphs. (Attached)
The juices provided are: V8 Carrot Ginger Blend and V8 Original Blend
Noticing the HPLC graphs I saw no peaks for the Original Blend for B-Carotene.
However the Carrot Ginger Blend showed similar peaks --> Why is this reason?
Please explain in terms of Retention time and Area (Under Curve).
Thank You!
Chapter 12 Solutions
Fundamentals of General, Organic, and Biological Chemistry, Books a la Carte Plus Mastering Chemistry with Pearson eText -- Access Card Package (8th Edition)
Ch. 12.2 - Locate and identify the functional groups in (a)...Ch. 12.2 - Draw structures for molecules that fit the...Ch. 12.3 - Prob. 12.3PCh. 12.3 - There are two branched-chain isomers with the...Ch. 12.4 - Draw the following three isomers of C5H12 as...Ch. 12.4 - Prob. 12.6PCh. 12.4 - Prob. 12.7PCh. 12.4 - Draw both condensed and line structures for the...Ch. 12.5 - Which of the following structures represent the...Ch. 12.5 - Are the pairs of compounds shown below the same...
Ch. 12.6 - Identify each carbon in the molecule shown in...Ch. 12.6 - Prob. 12.12PCh. 12.6 - Prob. 12.13PCh. 12.6 - Draw and name alkanes that meet the following...Ch. 12.6 - Prob. 12.15KCPCh. 12.6 - Prob. 12.1CIAPCh. 12.6 - Prob. 12.2CIAPCh. 12.6 - Prob. 12.3CIAPCh. 12.8 - Prob. 12.1MRPCh. 12.8 - Prob. 12.2MRPCh. 12.8 - Prob. 12.16PCh. 12.8 - Write the structures of all singly chlorinated...Ch. 12.10 - Prob. 12.18PCh. 12.10 - Prob. 12.19PCh. 12.10 - What is wrong with the following names? It will be...Ch. 12.10 - Prob. 12.21KCPCh. 12.10 - Prob. 12.4CIAPCh. 12.10 - (a) What common produce items might you see...Ch. 12 - Convert the following models into line drawings...Ch. 12 - Prob. 12.23UKCCh. 12 - Prob. 12.24UKCCh. 12 - Give the IUPAC names for the following...Ch. 12 - Prob. 12.26UKCCh. 12 - What characteristics of carbon make possible the...Ch. 12 - Prob. 12.28APCh. 12 - Prob. 12.29APCh. 12 - Prob. 12.30APCh. 12 - For each of the following, give an example of a...Ch. 12 - Identify the highlighted functional groups in the...Ch. 12 - Identify the functional groups in the following...Ch. 12 - Propose structures for molecules that fit the...Ch. 12 - Prob. 12.35APCh. 12 - What requirement must be met for two compounds to...Ch. 12 - Prob. 12.37APCh. 12 - Prob. 12.38APCh. 12 - Prob. 12.39APCh. 12 - Prob. 12.40APCh. 12 - Give an example of a compound that meets the...Ch. 12 - (a)There are two isomers with the formula C4H10....Ch. 12 - Write condensed structures for the following...Ch. 12 - Prob. 12.44APCh. 12 - Prob. 12.45APCh. 12 - Which of the following pairs of structures are...Ch. 12 - Prob. 12.47APCh. 12 - Prob. 12.48APCh. 12 - Prob. 12.49APCh. 12 - What are the IUPAC names of the following alkanes?Ch. 12 - Prob. 12.51APCh. 12 - Write condensed structures for the following...Ch. 12 - Draw line structures for the following...Ch. 12 - Name the following cycloalkanes:Ch. 12 - Prob. 12.55APCh. 12 - Prob. 12.56APCh. 12 - Prob. 12.57APCh. 12 - Prob. 12.58APCh. 12 - Prob. 12.59APCh. 12 - Prob. 12.60APCh. 12 - Prob. 12.61APCh. 12 - Write the formulas of the four singly chlorinated...Ch. 12 - Write the formulas of the three doubly brominated...Ch. 12 - Identify the indicated functional groups in the...Ch. 12 - The line structure for pregabalin (Lyrica) is...Ch. 12 - Prob. 12.66CPCh. 12 - Prob. 12.67CPCh. 12 - Most lipsticks are about 70% castor oil and wax....Ch. 12 - Prob. 12.69CPCh. 12 - Prob. 12.70CPCh. 12 - Prob. 12.71CPCh. 12 - Which of the following structures represent the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate pH of a solution prepared by dissolving 1.60g of sodium acetate, in 88.5 mL of 0.10 M acetic acid. Assume the volume change upon dissolving the sodium acetate is negligible. Ka is 1.75 x 10^-5arrow_forwardShow a mechanism that leads to the opening of the ring below under acid-catalyzed conditions. Give the correct Fischer projection for this sugar.arrow_forwardWhat is the stereochemical relationship between B & C?arrow_forward
- Don't use ai or any chat gpt will dislike okk just use accurate information okkk okkk just solve full accurate. don't use guidelines okk just did it accurate 100% sure experts solve it correct complete solutions okkk follow all instructions requirements okkkarrow_forwardhow would you make this plot in excel?arrow_forwardwhat is the productarrow_forward
- Balance the following equation and list of coefficients in order from left to right. SF4+H2O+—-> H2SO3+HFarrow_forwardProblem 15 of 15 Submit Using the following reaction data points, construct Lineweaver-Burk plots for an enzyme with and without an inhibitor by dragging the points to their relevant coordinates on the graph and drawing a line of best fit. Using the information from this plot, determine the type of inhibitor present. 1 mM-1 1 s mM -1 [S]' V' with 10 μg per 20 54 10 36 20 5 27 2.5 23 1.25 20 Answer: |||arrow_forward12:33 CO Problem 4 of 15 4G 54% Done On the following Lineweaver-Burk -1 plot, identify the by dragging the Km point to the appropriate value. 1/V 40 35- 30- 25 20 15 10- T Км -15 10 -5 0 5 ||| 10 15 №20 25 25 30 1/[S] Г powered by desmosarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning


Anatomy & Physiology
Biology
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:OpenStax College

Biology (MindTap Course List)
Biology
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Cengage Learning


Principles Of Radiographic Imaging: An Art And A ...
Health & Nutrition
ISBN:9781337711067
Author:Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:Cengage Learning

GCSE Chemistry - Acids and Bases #34; Author: Cognito;https://www.youtube.com/watch?v=vt8fB3MFzLk;License: Standard youtube license