
Organic Chemistry
7th Edition
ISBN: 9780321803221
Author: Paula Y. Bruice
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.3, Problem 7P
Muscalure is the sex attractant of the common housefly. Flies are lured to traps filled with bait that contain muscalure and an insecticide. Eating the bait is fatal. How could you synthesize muscalure using 1-bromopentane as one of the starting materials?
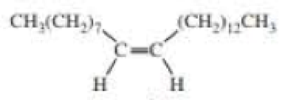
muscalure
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Show work....don't give Ai generated solution
Show work. don't give Ai generated solution
Don't used Ai solution
Chapter 12 Solutions
Organic Chemistry
Ch. 12.1 - Prob. 1PCh. 12.2 - Which is more reactive an organolithium compound...Ch. 12.2 - Prob. 3PCh. 12.3 - PROBLEM 6♦
Explain why tertiary alkyl halides...Ch. 12.3 - Muscalure is the sex attractant of the common...Ch. 12.3 - Prob. 8PCh. 12.3 - Prob. 9PCh. 12.3 - Prob. 10PCh. 12.4 - Prob. 13PCh. 12.4 - Prob. 14P
Ch. 12.4 - Prob. 15PCh. 12.4 - Show how the Suzuki and/or Heck reactions can be...Ch. 12.4 - Identify two pairs of an alkyl bromide and an...Ch. 12.5 - Prob. 19PCh. 12.5 - Draw the product of ring-closing metathesis for...Ch. 12.5 - Prob. 22PCh. 12 - Prob. 23PCh. 12 - Prob. 24PCh. 12 - Identify A through H.Ch. 12 - 26. Using the given starting material, any...Ch. 12 - Prob. 27PCh. 12 - Prob. 28PCh. 12 - Prob. 29PCh. 12 - Using ethynyleyclohexane as a starting material...Ch. 12 - Prob. 31PCh. 12 - Using the given starting material, any necessary...Ch. 12 - Prob. 33PCh. 12 - A student added an equivalent of...Ch. 12 - Using the given starting material, any necessary...Ch. 12 - Prob. 36PCh. 12 - Prob. 37PCh. 12 - Bombykol is the sex pheromone of the silk moth....Ch. 12 - Prob. 39PCh. 12 - A dibromide loses only one bromine when it reacts...Ch. 12 - What starting material is required in order to...Ch. 12 - Prob. 42PCh. 12 - Prob. 1PCh. 12 - Prob. 2PCh. 12 - Prob. 3PCh. 12 - Prob. 4P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Provide the correct common name for the compound shown here.arrow_forwardPh heat heatarrow_forward(12) Which one of the following statements about fluo- rometry is FALSE? a) Fluorescence is better detected at 90 from the exci- tation direction. b) Fluorescence is typically shifted to longer wave- length from the excitation wavelength. c) For most fluorescent compounds, radiation is pro- duced by a transitionarrow_forward
- Indicate the correct option.a) Graphite conducts electricity, being an isotropic materialb) Graphite is not a conductor of electricityc) Both are falsearrow_forward(f) SO: Best Lewis Structure 3 e group geometry:_ shape/molecular geometry:, (g) CF2CF2 Best Lewis Structure polarity: e group arrangement:_ shape/molecular geometry: (h) (NH4)2SO4 Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License