
Concept explainers
(a)
Interpretation: The Lewis and resonating structures of given compound PCl3is to be written and valence electrons are to be counted.
Concept introduction: Lewis structure shows sharing of electrons using dots. Each
(a)
Answer to Problem 4RQ
Sum of valence electrons of all atoms=26
Lewis structure with circles:
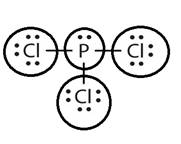
Explanation of Solution
Sum of valence electrons of all atoms=5+7+7+7=26
Lewis structure with circles:
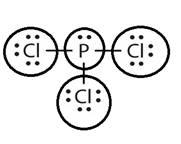
Phosphorus has five electrons and chlorine has seven electrons in valence shell. Thus, each chlorine atom shares one electron with central phosphorus atom to complete the octet.
From Lewis structure, octet of phosphorus and chlorine is complete.
There is no resonance in it.
(b)
Interpretation: The Lewis and resonating structures of given compound Br2is to be written and valence electrons are to be counted.
Concept introduction: Lewis structure shows sharing of electrons using dots. Each atom has number of dots equal to valence electrons. Every atom shares electrons to complete eight electrons in valence shell. Resonating structures are drawn when one structure can’t explain all the properties of molecule.
(b)
Answer to Problem 4RQ
Sum of valence electrons of all atoms=14
Lewis structure with circles:
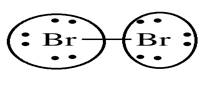
Explanation of Solution
Sum of valence electrons of all atoms=7+7=14
Lewis structure with circles:
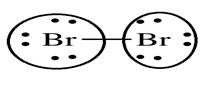
Bromine belongs to 17th group. So, it has seven electrons in its valence shell. It requires one electron to complete its octet thus share one electron with other bromine atom.
From Lewis structure, octet of bromine is complete.
There is no resonance in
(c)
Interpretation: The lewis and resonating structures of given compound
Concept introduction: Lewis structure shows sharing of electrons using dots. Each atom has number of dots equal to valence electrons. Every atom shares electrons to complete eight electrons in valence shell. Resonating structures are drawn when one structure can’t explain all the properties of molecule.
(c)
Answer to Problem 4RQ
Sum of valence electrons of all atoms=18
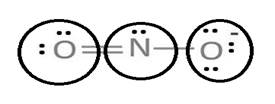
Lewis structure with circles:
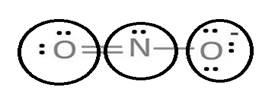
Resonating structure:
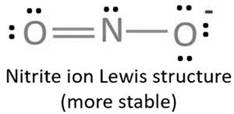
Explanation of Solution
Sum of valence electrons of all atoms=6+5+6+1=18
Lewis structure with circles:
Resonating structure:
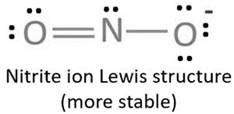
Lewis structure of nitrite ion shows nitrogen shares its one electron with oxygen atom carrying negative charge to complete its octet and two electrons with neutral oxygen atom to complete their octet.
But, experiment shows both the N-O bond lengths are equal. So, it has resonance in which negative charge is equally spread on both oxygen atoms and both N-O bond lengths lie in between single and double bond.
From Lewis structure, octet of both oxygen and nitrogen is complete.
(d)
Interpretation: The lewis and resonating structures of given compound O3 is to be written and valence electrons are to be counted.
Concept introduction: Lewis structure shows sharing of electrons using dots. Each atom has number of dots equal to valence electrons. Every atom shares electrons to complete eight electrons in valence shell. Resonating structures are drawn when one structure can’t explain all the properties of molecule.
(d)
Answer to Problem 4RQ
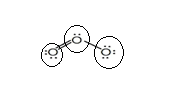
Sum of valence electrons of all atoms=18
Lewis structure with circles:
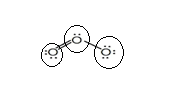
Resonating structure:
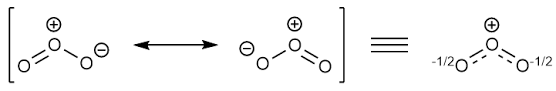
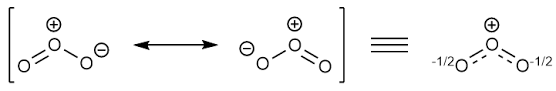
Explanation of Solution
Sum of valence electrons of all atoms=6+6+6=18
Lewis structure with circles:
Resonating structure:
The formula of ozone is
From Lewis structure, octet of all oxygen is complete.
(e)
Interpretation: The lewis and resonating structures of given compound is to be written and valence electrons are to be counted.
Concept introduction: Lewis structure shows sharing of electrons using dots. Each atom has number of dots equal to valence electrons. Every atom shares electrons to complete eight electrons in valence shell. Resonating structures are drawn when one structure can’t explain all the properties of molecule.
(e)
Answer to Problem 4RQ
Sum of valence electrons of all atoms=10
Lewis structure with circles:
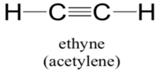
Explanation of Solution
Sum of valence electrons of all atoms=4+4+1+1=10
Lewis structure with circles:
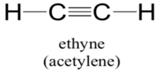
Carbon atom has four electrons in valence shell. It shares one electron with hydrogen atom to complete doublet of hydrogen. Now, to complete octet it shares three electrons with other carbon atom to complete octet and thus forms triple bond.
From Lewis structure, octet of all carbon is complete and doublet of hydrogen is complete.
(f)
Interpretation: The lewis and resonating structures of given compound is to be written and valence electrons are to be counted.
Concept introduction: Lewis structure shows sharing of electrons using dots. Each atom has number of dots equal to valence electrons. Every atom shares electrons to complete eight electrons in valence shell. Resonating structures are drawn when one structure can’t explain all the properties of molecule.
(f)
Answer to Problem 4RQ
Sum of valence electrons of all atoms=18
Lewis structure with circles:
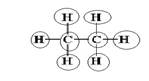
Explanation of Solution
Sum of valence electrons of all atoms=4+4+1+1+1+1+1+1=18
Lewis structure with circles:
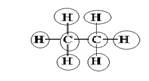
Carbon has four electrons in valence shell. So, each carbon forms one single bond with other carbon atom and three single bonds with three hydrogen atoms.
From Lewis structure, octet of all carbon is complete and doublet of hydrogen is complete.
(g)
Interpretation: The lewis and resonating structures of given compound is to be written and valence electrons are to be counted.
Concept introduction: Lewis structure shows sharing of electrons using dots. Each atom has number of dots equal to valence electrons. Every atom shares electrons to complete eight electrons in valence shell. Resonating structures are drawn when one structure can’t explain all the properties of molecule.
(g)
Answer to Problem 4RQ
Sum of valence electrons of all atoms=10
Lewis structure with circles:
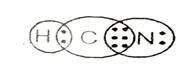
Explanation of Solution
Sum of valence electrons of all atoms=1+4+5=10
Lewis structure with circles:
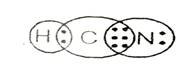
Lewis structure shows there is single bond between carbon and hydrogen and triple bond between carbon and nitrogen.
From Lewis structure, octet of nitrogen and carbon is complete and doublet of hydrogen is complete.
(h)
Interpretation: The lewis and resonating structures of given compound is to be written and valence electrons are to be counted.
Concept introduction: Lewis structure shows sharing of electrons using dots. Each atom has number of dots equal to valence electrons. Every atom shares electrons to complete eight electrons in valence shell. Resonating structures are drawn when one structure can’t explain all the properties of molecule.
(h)
Answer to Problem 4RQ
Sum of valence electrons of all atoms=23
Lewis structure with circles:
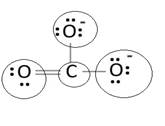
Resonating structure:
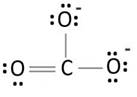
Explanation of Solution
Sum of valence electrons of all atoms=4+6+6+6+1=23
Lewis structure with circles:
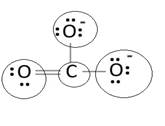
Resonating structure:
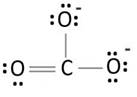
Lewis structure of carbonate ion shows one oxygen atom is bonded to carbon by double bond and two oxygen ions are bonded by single bonded. But in actual all the C-O bond lengths in carbonate ion is same. So, resonating structures of carbonate ions are formed. In resonance hybrid two unit negative charge is equally spread on three oxygen atoms and bond length of C-O lies in between double and single bond.
Lewis structure shows octet of oxygen and carbon is complete.
Chapter 12 Solutions
World of Chemistry, 3rd edition
- Please complete the reactions, thank youarrow_forwardConsider the synthesis. What is compound Y? Please explain what is happening in this question. Provide a detailed explanation and a drawing to show how the compound Y creates the product. The correct answer is D.arrow_forwardWhat would be the major product of the following reaction? Please include a detailed explanation of what is happening in this question. Include steps and a drawing to show this reaction proceeds and how the final product is formed. The correct answer is B. I put answer D and I don't really understand what is going on in the question.arrow_forward
- What is the product of the following reaction? Please explain what is happening in this question. Provide a detailed explanation and a drawing showing how the reagent is reacting with the catalysts to product the correct product. The correct answer is B.arrow_forwardWhat is the missing intermediate 1 and the final product 2. Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediate and how it occurs and how the final product is former.arrow_forwardWhat would be the reagents and conditions above and below the arrow that will complete the proposed acetoacetic ester synthesis? If it cannot be done efficiently, then I will choose that answer. There could be 2 or 4 reagents involved. Please provide a detailed explanation and drawings showing how it would proceed with the correct reagents.arrow_forward
- For benzene, the ∆H° of vaporization is 30.72 kJ/mol and the ∆S° of vaporization is 86.97 J/mol・K. At 1.00 atm and 228.0 K, what is the ∆G° of vaporization for benzene, in kJ/mol?arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reaction. it is spontaneous only at High T, it is spontaneous at low T it is nonspontaneous at all T it is spontanrous at all T. it is non spontaneous only at low T.arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forward
- Which of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forwardWhich of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forwardWhat is the major product of the following reaction? O O OH OH 1. BH 2. H₂O₂, NaOH OH OHarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





