
Classify the carbon atoms in each compound as 1°, 2°, 3°, or 4°.
a.
b.
(a)
Interpretation:
The carbon in the following compound should be classified as
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
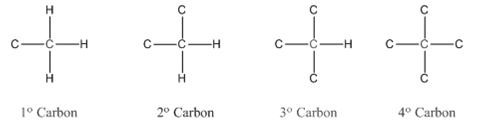
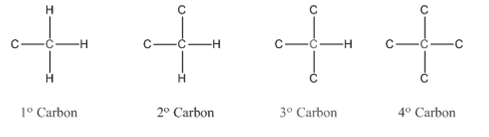
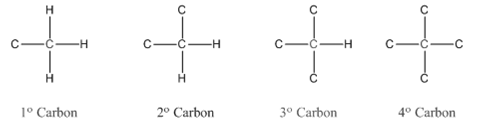
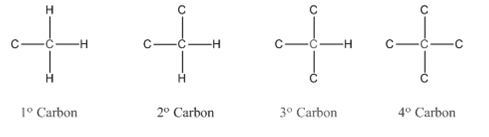
In organic chemistry, carbons are classified as primary, secondary, tertiary and quaternary carbons.
Primary carbon (
Secondary carbon (
Tertiary carbon (
Quaternary carbon (
Answer to Problem 12.3PP
Explanation of Solution
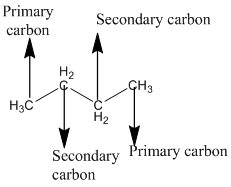
The given compound is
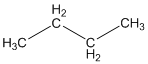
According to the above structure of compound, the terminal carbons present on both sides of the chain is classified as primary carbon as they are linked with one other carbon atom whereas carbon atoms which are present in between the terminal carbonsof the chain are linked with two other carbon atoms thus, classified as secondary carbon.
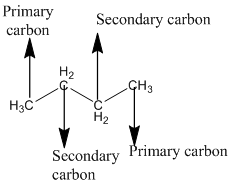
Thus, two primary and two secondary carbons are present in the structure.
(b)
Interpretation:
The carbon in the following compound should be classified as
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
In organic chemistry, carbons are classified as primary, secondary, tertiary and quaternary carbons.
Primary carbon (
Secondary carbon (
Tertiary carbon (
Quaternary carbon (
Answer to Problem 12.3PP
Explanation of Solution
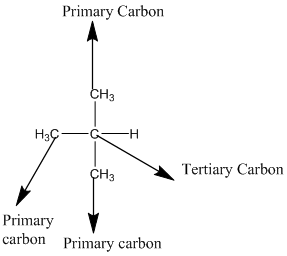
The given compound is
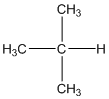
According to the above structure of compound, the terminal carbons (3 carbons) present is classified as primary carbon as they are linked with one other carbon atom whereas carbon atom which is present in the middle linked with three other carbon atoms thus, classified as tertiary carbon.
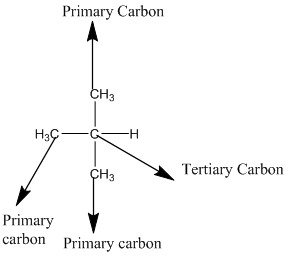
Thus, three primary carbons and onetertiary carbon atom are present in the structure.
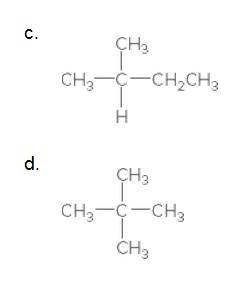
(c)
Interpretation:
The carbon in the following compound should be classified as
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
In organic chemistry, carbons are classified as primary, secondary, tertiary and quaternary carbons.
Primary carbon (
Secondary carbon (
Tertiary carbon (
Quaternary carbon (
Answer to Problem 12.3PP
Explanation of Solution
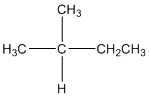
The given compound is
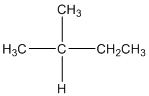
According to the above structure of compound, the terminal carbons (3 carbons) present is classified as primary carbon as they are linked with one other carbon atom whereas the carbon which is linked with two other carbon atoms is classified as secondary carbon and the carbon which is linked with three other carbon atoms is classified as tertiary carbon.
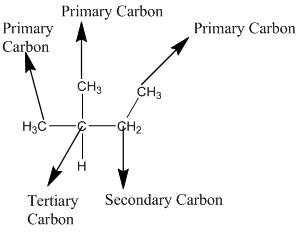
Thus, three primary carbons, one secondary carbon atom and one tertiary carbon atom are present in the structure.
(d)
Interpretation:
The carbon in the following compound should be classified as
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
In organic chemistry, carbons are classified as primary, secondary, tertiary and quaternary carbons.
Primary carbon (
Secondary carbon (
Tertiary carbon (
Quaternary carbon (
Answer to Problem 12.3PP
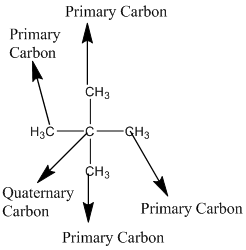
Explanation of Solution
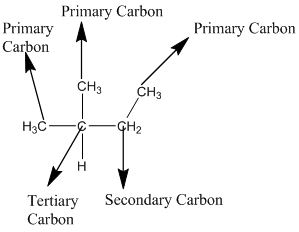
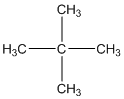
The given compound is
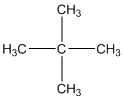
According to the above structure of compound, the terminal carbons (four carbons) present is classified as primary carbon as they are linked with one other carbon atom whereas the carbon which is linked with four other carbon atoms is classified as quaternary carbon.
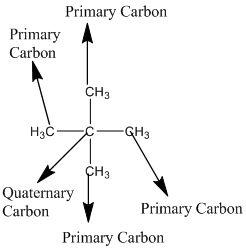
Thus, four primary carbons and one quaternary carbon atom are present in the structure.
Want to see more full solutions like this?
Chapter 12 Solutions
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
- I need help working this problem out step by step, I was trying to use my example from the txt book but all I know how to do is set it up. I need to be shown step by step as I am a visual learner. Please help me.arrow_forwardDon't used hand raitingarrow_forwardDon't used Ai solution and don't used hand raitingarrow_forward
- & Calculate the molar enthalpy of combustion (A combH) of 1.80 g of pyruvic acid (CH3COCOOH; 88.1 g mol-1) at 37 °C when they are combusted in a calorimeter at constant volume with a calorimeter constant = 1.62 kJ °C-1 and the temperature rose by 1.55 °C. Given: R = 8.314 J mol −1 °C-1 and the combustion reaction: AN C3H4O3 + 2.502(g) → 3CO2(g) + 2H2O(l)arrow_forwardAn unknown salt, AB, has the following precipitation reaction:A+(aq) + B-(aq) ⇌ AB(s) the K value for this reaction is 4.50 x10-6. Draw a model that represents what will happen when 1.00 L each of 1.00 M solution of A+(aq) and 1.00M solution of B-(aq) are combined.arrow_forward5. a) Use the rules in Example 4.4 (p. 99) and calculate sizes of octahedral and tetrahedral cavities in titanium and in zirconium. Use values for atomic radii given in Fig. 9.1 (p.291). (3 points) b) Consider the formation of carbides (MC) of these metals. Which metal is able to accommodate carbon atoms better, and which cavities (octahedral or tetrahedral) would be better suited to accommodate C atoms into metal's lattice? (4 points)arrow_forward
- 2. Read paragraph 3.4 in your textbook ("Chiral Molecules"), and explain if Cobalt(ethylenediamine) 33+ shown in previous problem is a chiral species. If yes, draw projections of both enantiomers as mirror images, analogous to mirror projections of hands (below). Mirror (4 points)arrow_forward3. Borane (BH3) belongs to D3h point group. Consider the vibrational (stretching) modes possible for B-H bonds under D3h symmetry. Using the methods we used in class, construct the reducible representation I, and break it down into irreducible representations using the character table provided. Sketch those modes, indicate whether they are IR-active. (6 points) D3h E 2C3 3C2 σh 283 30% A₁' 1 1 1 1 1 1 x² + y², z² 1 -1 1 1 -1 R₂ E' 2 0 2 0 (x, y) (x² - y², xy) " A₁" 1 1 -1 A2" 1 -1 -1 1 Z E" 2 -1 0 -2 1 0 (Ry, Ry) (xz, yz)arrow_forward1. List all the symmetry elements, and assign the compounds to proper point groups: a) HCIBrC-BrCIH Cl Br H (2 points) H Br b) Pentacarbonylmanganese(I)bromide Br OEC-Mn-CEO 00- c) Phenazine (aromatic molecule, with delocalized bonding) 1 d) Cobalt(ethylenediamine)33+ (just the cation) 3+ H₂N H₂ .NH2 (CI)3 NH2 H2 H₂N. (2 points) (2 points) (2 points)arrow_forward
- Hello, I desperately need help figuring out 8-14; I also wanted to see if you would mind letting me know if I picked the right degree as my melting points on the two graphs. Please and thank you in advance! All the information is provided.arrow_forwardThe reaction: A + B ⇌ 2 C, can be represented by the equilibrium expression, KC =[C]2[A][B]=258 at 520K.When 1.00 M of C was allowed to reach equilibrium and 0.055 M of A was formed. If this reaction wasperformed at the same temperature using 0.500 M C, what would the equilibrium concentration of Abe?arrow_forward1. What is the functional group of an alcohol and a phenol? 2. Why are some alcohols soluble in water? 3. Classify each of the following alcohols as primary, secondary or tertiary. a. 3-pentanol b. 2-methyl-2-butanol c. 1-propanolarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





