
Concept explainers
(a)
Interpretation:
An efficient synthesis that can be used to achieve each of the following transformations is to be determined.
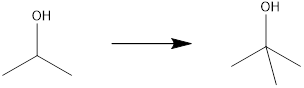
Concept introduction:
The product molecule has one more carbon atom than the starting material. For the generation of tertiary alcohol from secondary alcohol, there must be oxidation of alcohol to the carbonyl group, after which, there must be an addition of a methyl fragment, preferably using a Grignard reagent. This after hydrolysis can yield the desired tertiary alcohol.
(b)
Interpretation:
An efficient synthesis that can be used to achieve each of the following transformations is to be determined.

Concept introduction: The final product has one more carbon atom than the starting material. This can be facilitated by the addition of a methyl group, preferably using a Grignard reagent after conversion of
(c)
Interpretation:
The efficient synthesis that can be used to achieve the following transformation is to be determined.
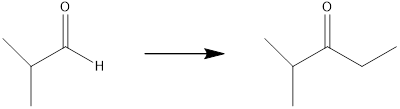
Concept introduction:
The final product contains two additional carbon atoms, so the synthesis requires a carbon-carbon bond-forming reaction. To facilitate this, the desired product should be produced by oxidizing the generated alcohol using the proper Grignard reagent.
(d)
Interpretation:
The efficient synthesis that can be used to achieve each of the following transformations is to be determined.
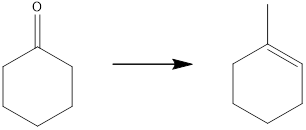
Concept introduction:
The target molecule contains one additional carbon atom, so the synthesis must have a carbon-carbon bond-forming reaction. This can be achieved by using a Grignard reagent followed by an elimination reaction which can yield the desired unsaturated product.
(e)
Interpretation:
The efficient synthesis that can be used to achieve each of the following transformations is to be determined.

Concept introduction:
The target molecule contains three additional carbon atoms, so the reaction needs a Carbon−Carbon bond-forming reaction. The
(f)
Interpretation:
An efficient synthesis that can be used to achieve each of the following transformations is to be determined.
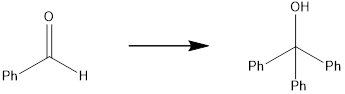
Concept introduction:
The target molecule has two additional phenyl groups, which can be done by adding Grignard reagent containing phenyl groups two times, followed by oxidation. The starting material contains carbonyl carbon which is the reaction center for the phenyl part of the Grignard reagent.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

