
CONCEPTUAL INTEGRATED SCIENCE (PEARSON+
3rd Edition
ISBN: 2818440059223
Author: Hewitt
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 12, Problem 72TE
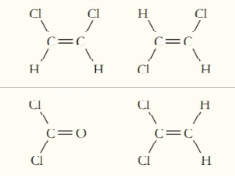
Circle the molecule from each pair that should have a higher boiling point (atomic numbers):
(a)
(b)
(c)
(d)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Y!mobile 4G
10:51 PM
43%
Messenger
cdn.fbsbx.com
Done
FLUID- is substance whose shape can easily change and is able to flow. Offer little resistance to change in shape when
Rothe gases and liquids are fluids.
11 of 12 fmolecules that randomly arranged and held together by weak cohesive forces and by forces exert-
ainer.
On pages 209-221 of the General Physics 1 textbook, read and analyze the key concepts, equations and problem
solving strategies of Fluid Mechanics.
IV. SIMPLE ACTIVITY
Activity 1
Directions: Read, analyze and solve the problem below. Show your complete solution then box your final answer.
A uniform silver sphere and a uniform gold sphere have the same mass. What is the ratio of the radius of the silver
sphere to the radius of the gold sphere?
Activity 2
Directions: Read, analyze and solve the problem below. Show your complete solution then box your final answer.
Joel watches his fish tank and notices that the angel fish likes to feed at the water's surface, while the…
No Chatgpt please will upvote
Don't use ai to answer I will report you answer
Chapter 12 Solutions
CONCEPTUAL INTEGRATED SCIENCE (PEARSON+
Ch. 12 - How many electrons can occupy the first shell? How...Ch. 12 - Which electrons are represented by an electron-dot...Ch. 12 - Prob. 3RCCCh. 12 - How does an ion differ from an atom?Ch. 12 - To become a negative ion, does an atom lose or...Ch. 12 - Prob. 6RCCCh. 12 - Prob. 7RCCCh. 12 - Prob. 8RCCCh. 12 - Prob. 9RCCCh. 12 - Prob. 10RCC
Ch. 12 - Prob. 11RCCCh. 12 - Prob. 12RCCCh. 12 - Why do nonpolar substances boil at relatively low...Ch. 12 - Which has a greater degree of symmetry-a polar...Ch. 12 - Why dont oil and water mix?Ch. 12 - Prob. 16RCCCh. 12 - What is a hydrogen bond?Ch. 12 - Are induced dipoles permanent?Ch. 12 - What happens to the volume of a sugar solution as...Ch. 12 - Prob. 20RCCCh. 12 - Is concentration typically given with the volume...Ch. 12 - Why does the solubility of a gas solute in a...Ch. 12 - Why do sugar crystals dissolve faster when...Ch. 12 - Is sugar a polar or nonpolar substance?Ch. 12 - Do metals more readily gain or lose electrons?Ch. 12 - What is an alloy?Ch. 12 - What is a native metal?Ch. 12 - Prob. 28TISCh. 12 - Prob. 29TISCh. 12 - How is a solution different from a suspension?Ch. 12 - Prob. 36TCCh. 12 - Prob. 37TCCh. 12 - Rank the following in order of increasing...Ch. 12 - Rank the following in order of decreasing boiling...Ch. 12 - Rank these solutions in order of increasing...Ch. 12 - Rank the following compounds in order of...Ch. 12 - Prob. 42TSCh. 12 - Prob. 43TSCh. 12 - Prob. 44TSCh. 12 - How much sodium chloride, in grams, is needed to...Ch. 12 - If water is added to 1mole of sodium chloride in a...Ch. 12 - Prob. 47TECh. 12 - Prob. 48TECh. 12 - How many more electrons can fit within the valence...Ch. 12 - Prob. 50TECh. 12 - What happens when hydrogens electron gets close to...Ch. 12 - Why does an atom with few valence electrons tend...Ch. 12 - Why it is so easy for a magnesium atom to lose two...Ch. 12 - Why doesnt the neon atom tend to lose or gain any...Ch. 12 - Sulfuric acid, H2SO4, loses two protons to form...Ch. 12 - Prob. 56TECh. 12 - Which should be more difficult to pull apart: a...Ch. 12 - Given that the total number of atoms on our planet...Ch. 12 - Prob. 59TECh. 12 - Two fluorine atoms join together to form a...Ch. 12 - How are metallic bonds similar to ionic bonds? How...Ch. 12 - What drives an atom to form a covalent bond: its...Ch. 12 - Atoms of nonmetallic elements form covalent bonds,...Ch. 12 - Prob. 64TECh. 12 - Prob. 65TECh. 12 - Prob. 66TECh. 12 - In each molecule, which atom carries the greater...Ch. 12 - Which is more polar: a sulfur-bromineS-Br bond or...Ch. 12 - True or False: The greater the nuclear charge of...Ch. 12 - True or False: The more shells in an atom, the...Ch. 12 - Water, H2O, and methane, CH4, have about the same...Ch. 12 - Circle the molecule from each pair that should...Ch. 12 - Three kids sitting equally apart around a table...Ch. 12 - Why is the oxygen atom of a water molecule...Ch. 12 - Look to the molecules listed in Table 12.2. How...Ch. 12 - Which is stronger: the covalent bond that holds...Ch. 12 - The charges with sodium chloride are all...Ch. 12 - Prob. 78TECh. 12 - Prob. 79TECh. 12 - Why is calcium fluoride, CaF2, a high melting...Ch. 12 - Of the two structures shown here, one is a typical...Ch. 12 - Mixtures can be separated into their components by...Ch. 12 - Why cant the elements of a compound be separated...Ch. 12 - Many dry cereals are fortified with iron, which is...Ch. 12 - Classify the following as element, compound, or...Ch. 12 - Which of the following boxes best represents a...Ch. 12 - Which is more dense: air saturated with water...Ch. 12 - How many sugar molecules are there in a 2M sugar...Ch. 12 - Prob. 89TECh. 12 - Which should weigh more: 100mL of fresh water or...Ch. 12 - Prob. 91TECh. 12 - The boiling point of 1, 4-butanediol is 230C....Ch. 12 - Based on atomic size, which would you expect to be...Ch. 12 - If nitrogen, N2, were pumped into your lungs at...Ch. 12 - Prob. 95TECh. 12 - Account for the observation that ethanol, C2H5OH,...Ch. 12 - At 10C, which is more concentrated: a saturated...Ch. 12 - Why is rain or snow called precipitation?Ch. 12 - Hydrogen chloride HCl is a gas at room...Ch. 12 - Some bottled water is now advertised as containing...Ch. 12 - Two plastic bottles of fresh seltzer water are...Ch. 12 - Would you expect to find more dissolved oxygen in...Ch. 12 - What should be done with mining pits after all...Ch. 12 - What are some of the obstacles people face when...Ch. 12 - Oxygen, O2, dissolves quite well within a class of...Ch. 12 - Prob. 1RATCh. 12 - Prob. 2RATCh. 12 - Why are ores so valuable? a They are sources of...Ch. 12 - In terms of the periodic table, is there an abrupt...Ch. 12 - When nitrogen and fluorine combine to form a...Ch. 12 - Prob. 6RATCh. 12 - Someone argues that you shouldnt drink tap water...Ch. 12 - Prob. 8RATCh. 12 - Fish dont live very longer in water that has just...Ch. 12 - Prob. 10RAT
Additional Science Textbook Solutions
Find more solutions based on key concepts
33. Consider the reaction:
The tabulated data were collected for the concentration of C4H8 as a function...
Chemistry: Structure and Properties (2nd Edition)
Plants use the process of photosynthesis to convert the energy in sunlight to chemical energy in the form of su...
Campbell Essential Biology with Physiology (5th Edition)
4. How do gross anatomy and microscopic anatomy differ?
Human Anatomy & Physiology (2nd Edition)
Plants use the process of photosynthesis to convert the energy in sunlight to chemical energy in the form of su...
Campbell Essential Biology (7th Edition)
Level 2: Application/Analysis 4. Nitrifying bactcria participatc in the nitrogen cycle mainly by (A) converting...
Campbell Biology (11th Edition)
Which coastal area experiences the largest tidal range difference in height between the high tide and low tide?...
Applications and Investigations in Earth Science (9th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Don't use chat gpt It Chatgpt means downvotearrow_forwardNo Chatgpt pleasearrow_forwardConsider a pure sample of a radioactive isotope with a mass number of (50). If the sample has mass of (25.0) micrograms and the isotope has a half-life of (17.5)x106 years, determine the decay rate for the sample. Give your answer in decays/second and with 3 significant figures.arrow_forward
- A = 13, B = 04, C = 4 A particular radioactive isotope has a half-life of (29.8) years. If the initial amount of the isotope was (28.5) g, how years later will the only (7.20) g remain of this isotope? Give your answer in years and with 3 significant figures.arrow_forwardA particular radioactive isotope has a half-life of (6.5) hours. If you have (24.5) g of the isotope at 10:00 AM, how much will you have at 7:30PM? Give your answer in grams (g) and with 3 significant figures.arrow_forwardSOLVE STEP BY STEP WITHOUT ARTIFICIAL INTELLIGENCE A ship is located in a certain region of the ocean, conducting research that requires knowledge of the sea depth at that point. To do so, it emits a signal with a wavelength of 40 m and a frequency of 30 Hz. If the signal is detected by the ship's radar 8 seconds later, what is the depth of the sea in that region?arrow_forward
- No Chatgpt please will upvotearrow_forwardIf ur using Chatgpt leave this problem otherwise will downvotearrow_forwardFor the following circuit, consider the resistor values given in the table and that it is powered by a battery having a fem of ε= 10.0 V and internal resistance r= 1.50 Ω. Determine:(a)Equivalent resistance from points a and b.b)Potential difference of EACH of the seven resistors.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Classical Dynamics of Particles and SystemsPhysicsISBN:9780534408961Author:Stephen T. Thornton, Jerry B. MarionPublisher:Cengage Learning
Classical Dynamics of Particles and SystemsPhysicsISBN:9780534408961Author:Stephen T. Thornton, Jerry B. MarionPublisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning

University Physics Volume 3
Physics
ISBN:9781938168185
Author:William Moebs, Jeff Sanny
Publisher:OpenStax

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Classical Dynamics of Particles and Systems
Physics
ISBN:9780534408961
Author:Stephen T. Thornton, Jerry B. Marion
Publisher:Cengage Learning

Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning

The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY