
Concept explainers
(a)
Interpretation:
Density of the liquid carbon dioxide is greater or less than solid carbon dioxide has to be identified.
Concept introduction:
All three phases of
(a)
Answer to Problem 33PS
The density of liquid
Explanation of Solution
The phase diagram of
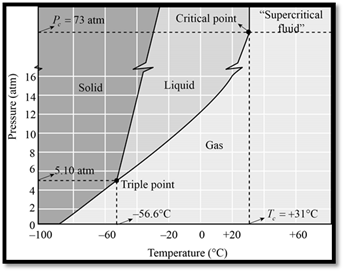
Figure 1
When seeing the phase diagram of
(b)
Interpretation:
Phase at which carbon dioxide at 5tm and
Concept introduction:
All three phases of
(b)
Answer to Problem 33PS
Explanation of Solution
The phase diagram of
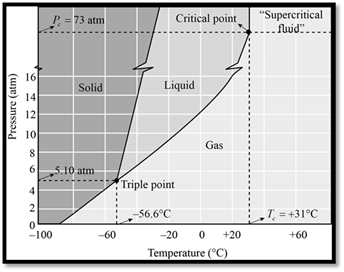
Figure 1
When seeing the phase diagram of
In the phase diagram of
(b)
Interpretation:
Carbon dioxide can be liquefied at
Concept introduction:
All three phases of
(b)
Answer to Problem 33PS
Explanation of Solution
The phase diagram of
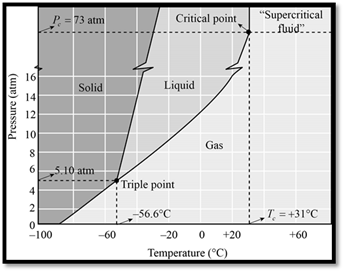
Figure 1
When seeing the phase diagram of
In the phase diagram of
Want to see more full solutions like this?
Chapter 12 Solutions
Chemistry & Chemical Reactivity, Hybrid Edition (with OWLv2 24-Months Printed Access Card)
- Can I please get help with this?arrow_forwardUse the Henderson-Hasselbalch equation to calculate pH of a buffer containing 0.050M benzoic acidand 0.150M sodium benzoate. The Ka of benzoic acid is 6.5 x 10-5arrow_forwardA. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forward
- What is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
