
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.66P
Devise a synthesis of each compound from the indicated starting material, organic compounds containing one or two carbons, and any other required reagents.
a. 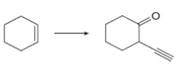 c.
c. 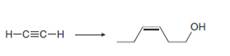
b. 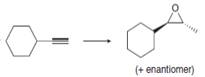 d.
d. 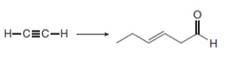
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Do you do chemistry assignments
Using the conditions of spontaneity to deduce the signs of AH and AS
Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy
AS.
Note: if you have not been given enough information to decide a sign, select the "unknown" option.
reaction
observations
conclusions
A
This reaction is always spontaneous, but
proceeds slower at temperatures above
120. °C.
ΔΗ is
(pick one)
AS is
(pick one)
ΔΗ is
(pick one)
B
This reaction is spontaneous except above
117. °C.
AS is
(pick one)
ΔΗ is
(pick one)
This reaction is slower below 20. °C than
C
above.
AS is
|(pick one)
?
18
Ar
1
Calculating the pH at equivalence of a titration
Try Again
Your answer is incorrect.
0/5
a
A chemist titrates 70.0 mL of a 0.7089 M hydrocyanic acid (HCN) solution with 0.4574M KOH solution at 25 °C. Calculate the pH at equivalence. The pK of
hydrocyanic acid is 9.21.
Round your answer to 2 decimal places.
Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of KOH solution added.
pH
=
11.43]
G
00.
18
Ar
B•
Chapter 12 Solutions
Organic Chemistry
Ch. 12 - Prob. 12.1PCh. 12 - Problem 12.2 What alkane is formed when each...Ch. 12 - Prob. 12.3PCh. 12 - Prob. 12.4PCh. 12 - Prob. 12.5PCh. 12 - Prob. 12.6PCh. 12 - Compound Molecular formula before...Ch. 12 - Problem 12.8 Draw the products formed when...Ch. 12 - Prob. 12.9PCh. 12 - Prob. 12.10P
Ch. 12 - Problem 12.11 (a) Draw the structure of a compound...Ch. 12 - Prob. 12.12PCh. 12 - Prob. 12.13PCh. 12 - Problem 12.14 Draw the products of each...Ch. 12 - Prob. 12.15PCh. 12 - Problem 12.16 Draw all stereoisomers formed when...Ch. 12 - Prob. 12.17PCh. 12 - Problem 12.18 Draw the products formed when both...Ch. 12 - Problem 12.19 Draw the products formed when each...Ch. 12 - Prob. 12.20PCh. 12 - Prob. 12.21PCh. 12 - Problem 12.22 Draw the products formed when each...Ch. 12 - Prob. 12.23PCh. 12 - Problem 12.24 Draw the organic products in each of...Ch. 12 - Prob. 12.25PCh. 12 - Prob. 12.26PCh. 12 - Problem 12.27 Draw the products of each Sharpless...Ch. 12 - Prob. 12.28PCh. 12 - 12.29 Draw the products formed when A is treated...Ch. 12 - Prob. 12.30PCh. 12 - 12.31 Devise a synthesis of the following compound...Ch. 12 - Prob. 12.32PCh. 12 - Prob. 12.33PCh. 12 - Prob. 12.34PCh. 12 - Prob. 12.35PCh. 12 - Prob. 12.36PCh. 12 - 12.37 Stearidonic acid (C18H28O2) is an...Ch. 12 - Draw the organic products formed when cyclopentene...Ch. 12 - Draw the organic products formed when allylic...Ch. 12 - Draw the organic products formed in each reaction....Ch. 12 - Prob. 12.41PCh. 12 - Prob. 12.42PCh. 12 - Prob. 12.43PCh. 12 - What alkene is needed to synthesize each 1,2-diol...Ch. 12 - Prob. 12.45PCh. 12 - 12.46 (a)What product is formed in Step [1] of the...Ch. 12 - Draw the products formed after Steps 1 and 2 in...Ch. 12 - 12.48 Draw the products formed in each oxidative...Ch. 12 - What alkene or alkyne yields each set of products...Ch. 12 - Prob. 12.50PCh. 12 - Prob. 12.51PCh. 12 - Prob. 12.52PCh. 12 - Prob. 12.53PCh. 12 - 12.54 An unknown compound A of molecular formula ...Ch. 12 - 12.55 DHA is a fatty acid derived from fish oil...Ch. 12 - Prob. 12.56PCh. 12 - 12.57 Draw the product of each asymmetric...Ch. 12 - 12.58 Epoxidation of the following allylic alcohol...Ch. 12 - Prob. 12.59PCh. 12 - 12.60 Identify A in the following reaction...Ch. 12 - Prob. 12.61PCh. 12 - 12.62 It is sometimes necessary to isomerize a cis...Ch. 12 - 12.63 Devise a synthesis of each compound from...Ch. 12 - Prob. 12.64PCh. 12 - Prob. 12.65PCh. 12 - 12.66 Devise a synthesis of each compound from the...Ch. 12 - Prob. 12.67PCh. 12 - Prob. 12.68PCh. 12 - 12.69 Devise a synthesis of each compound from as...Ch. 12 - Prob. 12.70PCh. 12 - Prob. 12.71PCh. 12 - 12.72 Draw a stepwise mechanism for the following...Ch. 12 - Prob. 12.73PCh. 12 - Prob. 12.74PCh. 12 - 12.75 Sharpless epoxidation of allylic alcohol X...
Additional Science Textbook Solutions
Find more solutions based on key concepts
True or false? Some trails are considered vestigial because they existed long ago.
Biological Science (6th Edition)
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Describe the evolution of mammals, tracing their synapsid lineage from early amniote ancestors to true mammals....
Loose Leaf For Integrated Principles Of Zoology
What process causes the Mediterranean intermediate Water MIW to become more dense than water in the adjacent At...
Applications and Investigations in Earth Science (9th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Biological Macromolecules Naming and drawing the products of aldose oxidation and reduction aw a Fischer projection of the molecule that would produce L-ribonic acid if it were subjected to mildly oxidizing reaction conditions. Click and drag to start drawing a structure. X AP ‡ 1/5 Naor Explanation Check McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibilarrow_forward● Biological Macromolecules Identifying the parts of a disaccharide Take a look at this molecule, and then answer the questions in the table below it. CH2OH O H H H OH OH OH H H CH2OH H O OH H OH H H H H OH Is this a reducing sugar? Does this molecule contain a glycosidic bond? If you said this molecule does contain a glycosidic bond, write the symbol describing it. If you said this molecule does contain a glycosidic bond, write the common names (including anomer and enantiomer labels) of the molecules that would be released if that bond were hydrolyzed. If there's more than one molecule, separate each name with a comma. Explanation Check O yes X O no ○ yes O no Uarrow_forwardThe aim of the lab is to measure the sodium content from tomato sauce using the Mohr titration method. There are two groups being: Regular Tomato sauce & Salt Reduced tomato sauce QUESTION: State how you would prepare both Regular & Salt reduced tomato sauce samples for chemical analysis using the Mohr titration methodarrow_forward
- Using the conditions of spontaneity to deduce the signs of AH and AS Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A The reverse of this reaction is always spontaneous but proceeds faster at temperatures above -48. °C. ΔΗ is (pick one) ✓ AS is (pick one) B This reaction is spontaneous except below 114. °C but proceeds at a slower rate below 135. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is C This reaction is exothermic and proceeds faster at temperatures above -43. °C. (pick one) AS is (pick one) v Х 5 ? 18 Ararrow_forwardion. A student proposes the following Lewis structure for the perchlorate (CIO) io : :0: : Cl : - - : :0: ك Assign a formal charge to each atom in the student's Lewis structure. atom central O formal charge ☐ top O ☐ right O ☐ bottom O ☐ Cl ☐arrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure Yes. Is the proposed Lewis structure reasonable? Cl- : 2: :Z: :Z: N—N : 0: C C1: O CO No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ Yes. No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0". ☑arrow_forward
- Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions ΔΗ is (pick one) A This reaction is faster above 103. °C than below. AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous only above -9. °C. AS is (pick one) ΔΗ is (pick one) C The reverse of this reaction is always spontaneous. AS is (pick one) 18 Ararrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A The reverse of this reaction is always spontaneous but proceeds slower at temperatures below 41. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous except above 94. °C. AS is (pick one) This reaction is always spontaneous, but ΔΗ is (pick one) C proceeds slower at temperatures below −14. °C. AS is (pick one) Х 00. 18 Ar 무ㅎ B 1 1arrow_forwardDraw the product of the reaction shown below. Ignore inorganic byproducts. + H CH3CH2OH HCI Drawingarrow_forward
- please explain this in simple termsarrow_forwardK Most Reactive Na (3 pts) Can the metal activity series (shown on the right) or a standard reduction potential table explain why potassium metal can be prepared from the reaction of molten KCI and Na metal but sodium metal is not prepared from the reaction of molten NaCl and K metal? Show how (not). Ca Mg Al с Zn Fe Sn Pb H Cu Ag Au Least Reactivearrow_forward(2 pts) Why is O2 more stable as a diatomic molecule than S2?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY