
Concept explainers
What is the coordination number of each sphere in (a) a simple cubic cell, (b) a body-centered cubic cell, and (c) a face-centered cubic cell? Assume the spheres are all the same.
(a)
Interpretation:
The co-ordination number of each sphere in simple cubic unit cell, body-centered cubic unit cell and face-centered cubic unit cell have to be identified.
Concept Introduction:
The simplest and basic unit of a crystalline solid is known as unit cell. It is cubic in shape. It is the building block of crystalline solids. The unit cells repeat themselves to build a lattice. Crystalline solids consist of many of such lattices. There are three types of unit cell – simple cubic unit cell, body – centered cubic unit cell and face – centered cubic unit cell.
In packing of the components in a solid, the components are imagined as spheres. A simple cubic unit cell is the simplest form of a cubic unit cell. A cube has eight vertices, twelve edges and six faces. Similarly a cubic unit cell has eight vertices, twelve edges and six faces. If in a cubic unit cell, the components occupy only the eight vertices, then the unit cell is known as simple cubic unit cell. So, each simple cubic unit cell has
In a body – centered cubic unit cell is another type of unit cell in which atoms are arranged in all the eight vertices of the unit cell with one atom per vertex. Further one atom occupies the center of the cube. Thus the number of atoms per unit cell in BCC unit cell is,
In a face – centered cubic unit cell the atoms are arranged in all the eight vertices of the unit cell with one atom per vertex. Further all the six faces of a cubic unit cell are occupied with one atom per face. Thus the number of atoms per unit cell in FCC unit cell is,
Co-ordination number of an atom or sphere is termed as the number of spheres it touches in the unit cell.
Answer to Problem 12.26QP
Co-ordination number of simple cubic unit cell is six.
Explanation of Solution
In simple cubic unit cell each sphere at the corner of the cubic unit cell is shared by eight other spheres. Each sphere is surrounded by six spheres.
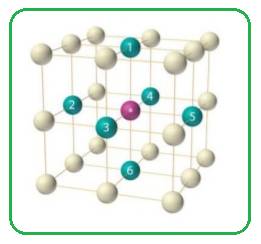
Figure 1
Thus the co-ordination number of simple cubic unit cell is six.
(b)
Interpretation:
The co-ordination number of each sphere in simple cubic unit cell, body-centered cubic unit cell and face-centered cubic unit cell have to be identified.
Concept Introduction:
The simplest and basic unit of a crystalline solid is known as unit cell. It is cubic in shape. It is the building block of crystalline solids. The unit cells repeat themselves to build a lattice. Crystalline solids consist of many of such lattices. There are three types of unit cell – simple cubic unit cell, body – centered cubic unit cell and face – centered cubic unit cell.
In packing of the components in a solid, the components are imagined as spheres. A simple cubic unit cell is the simplest form of a cubic unit cell. A cube has eight vertices, twelve edges and six faces. Similarly a cubic unit cell has eight vertices, twelve edges and six faces. If in a cubic unit cell, the components occupy only the eight vertices, then the unit cell is known as simple cubic unit cell. So, each simple cubic unit cell has
In a body – centered cubic unit cell is another type of unit cell in which atoms are arranged in all the eight vertices of the unit cell with one atom per vertex. Further one atom occupies the center of the cube. Thus the number of atoms per unit cell in BCC unit cell is,
In a face – centered cubic unit cell the atoms are arranged in all the eight vertices of the unit cell with one atom per vertex. Further all the six faces of a cubic unit cell are occupied with one atom per face. Thus the number of atoms per unit cell in FCC unit cell is,
Co-ordination number of an atom or sphere is termed as the number of spheres it touches in the unit cell.
Answer to Problem 12.26QP
Co-ordination number of Body-centered cubic unit cell is eight.
Explanation of Solution
There are two spheres per BCC unit cell. Each sphere touches eight other spheres.
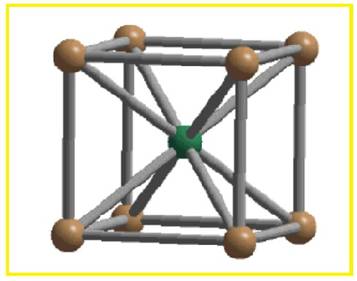
Figure 2
Thus the co-ordination number of body-centered cubic unit cell is eight.
(c)
Interpretation:
The co-ordination number of each sphere in simple cubic unit cell, body-centered cubic unit cell and face-centered cubic unit cell have to be identified.
Concept Introduction:
The simplest and basic unit of a crystalline solid is known as unit cell. It is cubic in shape. It is the building block of crystalline solids. The unit cells repeat themselves to build a lattice. Crystalline solids consist of many of such lattices. There are three types of unit cell – simple cubic unit cell, body – centered cubic unit cell and face – centered cubic unit cell.
In packing of the components in a solid, the components are imagined as spheres. A simple cubic unit cell is the simplest form of a cubic unit cell. A cube has eight vertices, twelve edges and six faces. Similarly a cubic unit cell has eight vertices, twelve edges and six faces. If in a cubic unit cell, the components occupy only the eight vertices, then the unit cell is known as simple cubic unit cell. So, each simple cubic unit cell has
In a body – centered cubic unit cell is another type of unit cell in which atoms are arranged in all the eight vertices of the unit cell with one atom per vertex. Further one atom occupies the center of the cube. Thus the number of atoms per unit cell in BCC unit cell is,
In a face – centered cubic unit cell the atoms are arranged in all the eight vertices of the unit cell with one atom per vertex. Further all the six faces of a cubic unit cell are occupied with one atom per face. Thus the number of atoms per unit cell in FCC unit cell is,
Co-ordination number of an atom or sphere is termed as the number of spheres it touches in the unit cell.
Answer to Problem 12.26QP
Co-ordination number of Face-centered cubic unit cell is twelve.
Explanation of Solution
There are four spheres per FCC unit cell. Each sphere is in contact with twelve spheres.
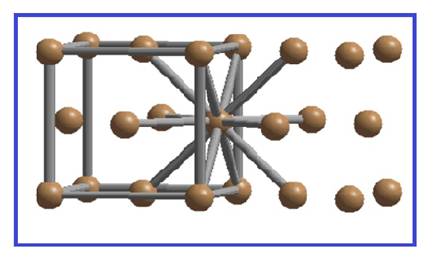
Figure 3
Thus the co-ordination number of simple cubic unit cell is twelve.
Want to see more full solutions like this?
Chapter 12 Solutions
Chemistry: Atoms First V1
- 2. 200 LOD For an unknown compound with a molecular ion of 101 m/z: a. Use the molecular ion to propose at least two molecular formulas. (show your work) b. What is the DU for each of your possible formulas? (show your work) C. Solve the structure and assign each of the following spectra. 8 6 4 2 (ppm) 150 100 50 ō (ppm) 4000 3000 2000 1500 1000 500 HAVENUMBERI-11arrow_forwardComplete the spectroscopy with structurearrow_forwardComplete the spectroscopy with structurearrow_forward
- Given the following concentrations for a system, calculate the value for the reaction quotient: Cl2(g)+ CS2(g) ⇌ CCl4(g)+ S2Cl2(g) Cl2 = 31.1 atm CS2 = 91.2 atm CCl4 = 2.12 atm S2Cl2 = 10.4 atmarrow_forwardMatch each chemical or item with the proper disposal or cleanup mwthod, Not all disposal and cleanup methods will be labeled. Metal sheets C, calcium, choroide solutions part A, damp metal pieces Part B, volumetric flask part A. a.Return to correct lables”drying out breaker. Place used items in the drawer.: Rinse with deionized water, dry as best you can, return to instructor. Return used material to the instructor.: Pour down the sink with planty of running water.: f.Pour into aqueous waste container. g.Places used items in garbage.arrow_forwardWrite the equilibrium constant expression for the following reaction: HNO2(aq) + H2O(l) ⇌ H3O+(aq) + NO2-(aq)arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





