
Loose Leaf For Introduction To Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259878084
Author: Smith Termodinamica En Ingenieria Quimica, J.m.; Van Ness, Hendrick C; Abbott, Michael; Swihart, Mark
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 12, Problem 12.13P
Problems 12.9 through 12.14 refer to the Txy diagram for ethanol(1)/ethyl acetate(2) shown in Fig.12.20.
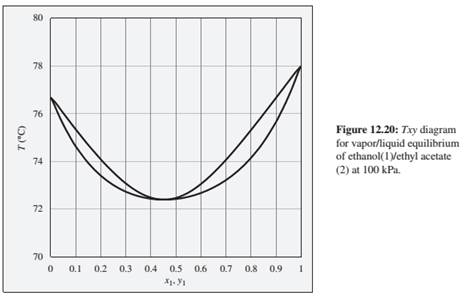
12.13. Consider an ethanol( 1 i/ethyl acetate(2) mixture with x1= 0.20. initially at 80°C and 100 kPa. Describe the evolution of phases and phase compositions as the temperature is gradually reduced to 70°C.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Topic: Production of propylene glycol from glycerol derived from palm oil.
QUESTION:Estimate capital items, operating costs and economics of the plant. Finally, report the estimatedreturn.The Detailed Factorial Method with approximately 25% accuracy must be used for detailedeconomic evaluation.Plant lifetime is fixed at 15 years.1) Depreciation and TaxesCalculate the depreciation using a suitable method (e.g., straight-line, declining balance) andincorporate tax implications based on current tax laws applicable to chemical plants.
Use following attached Process Flow Diagram as reference for this question.
Topic: Production of propylene glycol from glycerol derived from palm oil.
QUESTION:Estimate capital items, operating costs and economics of the plant. Finally, report the estimatedreturn.The Detailed Factorial Method with approximately 25% accuracy must be used for detailedeconomic evaluation.Plant lifetime is fixed at 15 years.1) Revenue EstimationEstimate the annual revenue based on the production capacity, product selling price, and marketdemand. Groups should also consider potential market fluctuations.
Use following attached Process Flow Diagram as reference for this question.
Topic: Production of propylene glycol from glycerol derived from palm oil.
QUESTION:Estimate capital items, operating costs and economics of the plant. Finally, report the estimatedreturn.The Detailed Factorial Method with approximately 25% accuracy must be used for detailedeconomic evaluation.Plant lifetime is fixed at 15 years.TASKS:1) Capital Cost EstimationProvide a detailed breakdown of the initial capital investment, including land, building,equipment, and installation costs. Include any assumptions made in the estimation. Use following attached Process Flow Diagram as reference for this question.
Chapter 12 Solutions
Loose Leaf For Introduction To Chemical Engineering Thermodynamics
Ch. 12 - Prob. 12.1PCh. 12 - Prob. 12.2PCh. 12 - Prob. 12.3PCh. 12 - Problems 12.3 through 12.8 refer to the Pxy...Ch. 12 - Problems 12.3 through 12.8 refer to the Pxy...Ch. 12 - Problems 12.3 through 12.8 refer to the Pxy...Ch. 12 - Problems 12.3 through 12.8 refer to the Pxy...Ch. 12 - Problems 12.3 through 12.8 refer to the Pxy...Ch. 12 - Problems 12.9 through 12.14 refer to the Txy...Ch. 12 - Problems 12.9 through 12.14 refer to the Txy...
Ch. 12 - Problems 12.9 through 12.14 refer to the Txy...Ch. 12 - Problems 12.9 through 12.14 refer to the Txy...Ch. 12 - Problems 12.9 through 12.14 refer to the Txy...Ch. 12 - Problems 12.9 through 12.14 refer to the Txy...Ch. 12 - Prob. 12.15PCh. 12 - Problems 12.16 through 12.21 refer to the Pxy...Ch. 12 - Problems 12.16 through 12.21 refer to the Pxy...Ch. 12 - Problems 12.16 through 12.21 refer to the Pxy...Ch. 12 - Problems 12.16 through 12.21 refer to the Pxy...Ch. 12 - Problems 12.16 through 12.21 refer to the Pxy...Ch. 12 - Prob. 12.21PCh. 12 - Problems 12.22 through 12.28 refer to the Txy...Ch. 12 - Problems 12.22 through 12.28 refer to the Txy...Ch. 12 - Problems 12.22 through 12.28 refer to the Txy...Ch. 12 - Problems 12.22 through 12.28 refer to the Txy...Ch. 12 - Problems 12.22 through 12.28 refer to the Txy...Ch. 12 - Problems 12.22 through 12.28 refer to the Txy...Ch. 12 - Prob. 12.28PCh. 12 - Problems 12.29 through 12.33 refer to the xy...Ch. 12 - Problems 12.29 through 12.33 refer to the xy...Ch. 12 - Problems 12.29 through 12.33 refer to the xy...Ch. 12 - Problems 12.29 through 12.33 refer to the xy...Ch. 12 - Problems 12.29 through 12.33 refer to the xy...Ch. 12 - Consider a binary liquid mixture for which the...Ch. 12 - Prob. 12.35PCh. 12 - Prob. 12.36P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Topic: Production of propylene glycol from glycerol derived from palm oil. QUESTION:Estimate capital items, operating costs and economics of the plant. Finally, report the estimatedreturn.The Detailed Factorial Method with approximately 25% accuracy must be used for detailedeconomic evaluation.Plant lifetime is fixed at 15 years.1) Breakeven Year CalculationUsing the cash flow analysis, calculate the breakeven year when the cumulative cash inflowequals the initial investment. Groups should graphically represent the breakeven point. Use following attached Process Flow Diagram as reference for this question.arrow_forwardTopic: Production of propylene glycol from glycerol derived from palm oil. QUESTION:Estimate capital items, operating costs and economics of the plant. Finally, report the estimatedreturn.The Detailed Factorial Method with approximately 25% accuracy must be used for detailedeconomic evaluation.Plant lifetime is fixed at 15 years.1) Cash Flow AnalysisDevelop a projected cash flow statement for the first 10 years of plant operation, consideringall the costs and revenues. Include working capital, loans, and interest payments if applicable. Use following attached Process Flow Diagram as reference for this question.arrow_forwardTopic: Production of propylene glycol from glycerol derived from palm oil.QUESTION:Estimate capital items, operating costs and economics of the plant. Finally, report the estimatedreturn.The Detailed Factorial Method with approximately 25% accuracy must be used for detailedeconomic evaluation.Plant lifetime is fixed at 15 years.1) Operational Cost AnalysisCalculate the yearly operational costs, including raw materials, labor, utilities, maintenance,and other recurring expenses. Provide a clear explanation of how these costs are derived. Use following attached Process Flow Diagram as reference for this question.arrow_forward
- Chemical Engineering Questionarrow_forwardA steam boiler or steam generator is a device used to produce steam by transferring heat to water. In our case, the combustion chamber is fueled with propane (C3H8) at a flowrate of 50.0 mol/h in an excess air of 50%. Assume that both propane and air are fed at 25ºC and the combustion gases leave the chamber at 200ºC. Pressure can be assumed to be atmospheric.* Determine: 1. The heat obtained assuming complete combustion. Compare the results using elements or compounds 2. The steam flowrate that could be generated if the heat is directed to obtain superheated steam at 2 bar and 160ºC from saturated liquid water at this pressure solvearrow_forwardIn a surface coating operation, a polymer (plastic) dissolved in liquid acetone is sprayed on a solid surface and a stream of hot air is then blown over the surface, vaporizing the acetone and leaving a residual polymer film of uniform thickness. Because environmental standards do not allow discharg- ing acetone into the atmosphere, a proposal to incinerate the stream is to be evaluated. The proposed process uses two parallel columns containing beds of solid particles. The air– acetone stream, which contains acetone and oxygen in stoichiometric proportion, enters one of the beds at 1500 mm Hg absolute at a rate of 1410 standard cubic meters per minute. The particles in the bed have been preheated and transfer heat to the gas. The mixture ignites when its temperature reaches 562 C, and combustion takes place rapidly and adiabatically. The combustion products then pass through and heat the particles in the second bed, cooling down to 350 C in the process. Period- ically the flow is…arrow_forward
- Propane is burned completely with excess oxygen. The product gas contains 24.5 mole% CO2, 6.10% CO, 40.8% H2O, and 28.6% O2. (a) Calculate the percentage excess O2 fed to the furnace. (b) A student wrote the stoichiometric equation of the combustion of propane to form CO2 and CO as: 2C3H8 + 11O2 → 3CO2 + 3CO + 8H2O According to this equation, CO2 and CO should be in a ratio of 1/1 in the reaction products, but in the product gas of Part (a) they are in a ratio of 24.8/6.12. Is that result possible? (Hint: Yes.) Explain howarrow_forwardEnumerate the various methods for catalyst preparation and discuss vividly any one of the methodsarrow_forward2. Design a spherical tank, with a wall thickness of 2.5 cm that will ensure that no more than 45 kg of hydrogen will be lost per year. The tank, which will operate at 500 °C, can be made from nickel, aluminum, copper, or iron (BCC). The diffusion coefficient of hydrogen and the cost per pound for each available material is listed in Table 1. Material Do (m2/s) Q (J/mol) Cost ($/kg) Nickel 5.5 x 10-7 37.2 16.09 Aluminium 1.6 x 10-5 43.2 2.66 Copper 1.1 x 10-6 39.3 9.48 Iron (BCC) 1.2 × 10-7 15.1 0.45 Table 1: Diffusion data for hydrogen at 500 °C and the cost of material.arrow_forward
- A flash drum at 1.0 atm is separating a feed consisting of methanol and water. If the feed rate is 2000 kg/h and the feed is 45 wt % methanol, what are the values of L (kg/h), V (kg/h), yM, xM (weight fractions), and Tdrum if 35% by weight of the feed is vaporized? VLE data are in Table 2-8.arrow_forwardQ1.B. Make a comparison between current control PWM rectifier in the abc reference frame and dq reference frame.arrow_forwardstep by steparrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The
Homogeneous and Heterogeneous Equilibrium - Chemical Equilibrium - Chemistry Class 11; Author: Ekeeda;https://www.youtube.com/watch?v=8V9ozZSKl9E;License: Standard YouTube License, CC-BY