
Concept explainers
(a)
Interpretation:
To write the mechanism for an uncatalyzed hydrolysis of methyl propionate.
Concept Introduction:
The hydrolysis reaction of ester is the reaction in which the breaking of ester bond is done by using water molecule in presence of acid or base as catalyst. The product obtained after the ester hydrolysis is the corresponding
The general reaction of an ester to produce in presence of only water is written as,
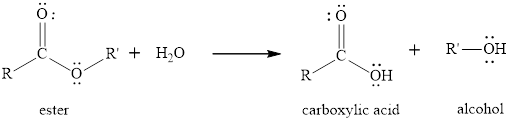
(b)
Interpretation:
To write the mechanism for aminolysis of phenyl formate using methyl amine.
Concept Introduction:
The reaction of a carboxylic acid derivative with ammonia, primary and secondary
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
EBK ESSENTIAL ORGANIC CHEMISTRY
- CHEMICAL KINETICS. Indicate the approximation methods for solving the rate equation.arrow_forwardTRANSMITTANCE เบบ Please identify the one structure below that is consistent with the 'H NMR and IR spectra shown and draw its complete structure in the box below with the protons alphabetically labeled as shown in the NMR spectrum and label the IR bands, including sp³C-H and sp2C-H stretch, indicated by the arrows. D 4000 OH LOH H₂C CH3 OH H₂C OCH3 CH3 OH 3000 2000 1500 HAVENUMBERI-11 1000 LOCH3 Draw your structure below and label its equivalent protons according to the peak labeling that is used in the NMR spectrum in order to assign the peaks. Integrals indicate number of equivalent protons. Splitting patterns are: s=singlet, d=doublet, m-multiplet 8 3Hb s m 1Hd s 3Hf m 2Hcd 2Had 1He 鄙视 m 7 7 6 5 4 3 22 500 T 1 0arrow_forwardRelative Transmittance 0.995 0.99 0.985 0.98 Please draw the structure that is consistent with all the spectral data below in the box and alphabetically label the equivalent protons in the structure (Ha, Hb, Hc ....) in order to assign all the proton NMR peaks. Label the absorption bands in the IR spectrum indicated by the arrows. INFRARED SPECTRUM 1 0.975 3000 2000 Wavenumber (cm-1) 1000 Structure with assigned H peaks 1 3 180 160 140 120 100 f1 (ppm) 80 60 40 20 0 C-13 NMR note that there are 4 peaks between 120-140ppm Integral values equal the number of equivalent protons 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0 fl (ppm)arrow_forward
- Calculate the pH of 0.0025 M phenol.arrow_forwardIn the following reaction, the OH- acts as which of these? NO2-(aq) + H2O(l) ⇌ OH-(aq) + HNO2(aq)arrow_forwardUsing spectra attached, can the unknown be predicted? Draw the predicition. Please explain and provide steps. Molecular focrmula:C16H13ClOarrow_forward
- Calculate the percent ionization for 0.0025 M phenol. Use the assumption to find [H3O+] first. K = 1.0 x 10-10arrow_forwardThe Ka for sodium dihydrogen phosphate is 6.32 x 10-8. Find the pH of a buffer made from 0.15 M H2PO4- and 0.25 M HPO42- .arrow_forwardThe Ka for lactic acid is 1.4 x 10-4. Find the pH of a buffer made from 0.066 M lactic acid and 0.088 M sodium lactate.arrow_forward
- Zaitsev's Rule 3) (a) Rank the following alkenes in order of decreasing stability. most stable A B C D > > > (b) Rank the following carbocations in order of decreasing stability least stable B C Darrow_forwardCalculate the pH of 0.25 M acetic acid.arrow_forwardCalculate the pH of 0.066 M ammonium ion.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

