
Concept explainers
(a)
Interpretation:
The reagent has to be identified for the conversion of methyl propanoate to the following compounds.
Concept introduction:
A reagent is a substance used to convert one chemical compound into another.
Transesterification: Transesterification reaction is an esterification reaction of ester react with excess of alcohol in the presence of either acid or base catalyst to form a new ester. The formation of one type of ester can be transformed in to other form of esters is called esterification when reaction moves forward when we use excess of an alcohol.

(b)
Interpretation:
The reagent has to be identified for the conversion of methyl propanoate to the following compounds.
Concept introduction:
Ester reaction with sodium hydroxide which goves the sodium salt and alcohol.
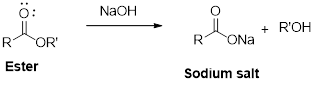
It is an example of saponification reaction.
(c)
Interpretation:
The reagent has to be identified for the conversion of methyl propanoate to the following compounds.
Concept introduction:
Amination reaction: Amination is the process by which an
(d)
Interpretation:
The reagent has to be identified for the conversion of methyl propanoate to the following compounds.
Concept introduction:
Ester reaction with sodium hydroxide which gives the sodium salt and alcohol this sodium salt further reaction with dil. Hydrochloric acid gives acid.

It is an example of saponification reaction.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
EBK ESSENTIAL ORGANIC CHEMISTRY
- The Ka for sodium dihydrogen phosphate is 6.32 x 10-8. Find the pH of a buffer made from 0.15 M H2PO4- and 0.25 M HPO42- .arrow_forwardThe Ka for lactic acid is 1.4 x 10-4. Find the pH of a buffer made from 0.066 M lactic acid and 0.088 M sodium lactate.arrow_forwardZaitsev's Rule 3) (a) Rank the following alkenes in order of decreasing stability. most stable A B C D > > > (b) Rank the following carbocations in order of decreasing stability least stable B C Darrow_forward
- Calculate the percent ionization for 0.35 M nitrous acid. Use the assumption to find [H3O+] first. K = 7.1 x 10-4arrow_forwardFor each of the following reactions: Fill in the missing reactant, reagent, or product (s), indicating stereochemistry where appropriate using dashed and wedged bonds. If the reaction forms a racemic mixture, draw both structures in the box and write the word “racemic”.arrow_forward5) Using the carbon-containing starting material(s), propose a synthesis based on the following retrosynthetic analysis. Provide structures for all intermediates. The carbon atoms in the product must originate from the starting material(s), but you may use as many equivalents of each starting material as you would like, and any reagent/reaction you know (note: no mechanisms are required). H H =arrow_forward
- Calculate the percent ionization for 0.0025 M phenol. Use the assumption to find [H3O+] first. K = 1.0 x 10-10arrow_forward10:04 AM Tue Mar 25 Sunday 9:30 AM 95% Edit Draw the corresponding structures in each of the boxes below: Ester Name Methyl butyrate (Example) Alcohol Structure H3C-OH Acid Structure Ester Structure Isoamyl acetate Ethyl butyrate Propyl acetate Methyl salicylate Octyl acetate Isobutyl propionate Benzyl butyrate Benzyl acetate Ethyl acetate H₂C OH HCarrow_forward2) For each of the following reactions: (i) (ii) Fill in the missing reactant, reagent, or product (s), indicating stereochemistry where appropriate using dashed and wedged bonds. If the reaction forms a racemic mixture, draw both structures in the box and write the word "racemic". (a) (b) 1) R₂BH 2) H₂O2, NaOH (aq) HBr Br racemic Br + Br Br racemicarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

