
EBK ESSENTIAL ORGANIC CHEMISTRY
3rd Edition
ISBN: 8220100659461
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11, Problem 39P
D. N. Kursanov, a Russian chemist, proved that the bond that is broken in the hydroxide-ion-promoted hydrolysis of an ester is the acyl C—O bond, rather than the alkyl C—O bond, by studying the hydrolysis of the following ester under basic conditions:
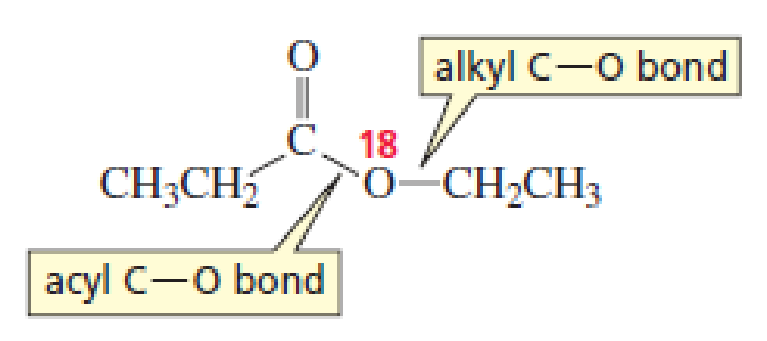
- a. What product contained the 18O label?
- b. What product would have contained the 18O label if the alkyl C—O bond had broken?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
3-Methylbutanoic acid, produced by bacteria from leucine, is a component of wine flavor and is responsible for foot odor.Which
alkylating agent(s) should be used for the malonic ester synthesis of 3-methylbutanoic acid?
O
O
O
A Ethyl bromide and methyl bromide
B 2-Bromopropane and methyl bromide
с
D
2-Bromopropane
Methyl bromide, methyl bromide, methyl bromide
E Methyl bromide, malonyl bromide
The reaction of a nitrile with an alcohol in the presence of a strong acid forms an N-substituted amide. This reaction, known as the Ritter reaction, doesnot work with primary alcohols.
a. Why does the Ritter reaction not work with primary alcohols?
b. Provide an explanation for why an amide is less susceptible to nucleophilic attack than its corresponding ester.
10. Which of the following compounds does not undergo hydrolysis with
either acid or basic catalysis:
00
H3Cca
CH3CH2ČNH2
CHICOCHCH3
CH3COCH2CH2CH3
B.
CHICCH2CH3
F. None of the molecules shown undergoes hydrolysis.
Chapter 11 Solutions
EBK ESSENTIAL ORGANIC CHEMISTRY
Ch. 11.1 - The aromas of many flowers and fruits are due to...Ch. 11.1 - Name the following compounds:Ch. 11.1 - Prob. 3PCh. 11.2 - Prob. 4PCh. 11.2 - Prob. 5PCh. 11.4 - a. What is the product of the reaction of acetyl...Ch. 11.4 - Prob. 7PCh. 11.5 - Using the pKa values listed in Table 11.1, predict...Ch. 11.6 - Starting with acetyl chloride, what neutral...Ch. 11.6 - Prob. 10P
Ch. 11.7 - Prob. 11PCh. 11.8 - Prob. 13PCh. 11.8 - Using the mechanism for the acidcatalyzed...Ch. 11.8 - Prob. 15PCh. 11.8 - Prob. 16PCh. 11.8 - Prob. 17PCh. 11.9 - Prob. 18PCh. 11.10 - Show how each of the following esters could be...Ch. 11.11 - Which of the following reactions would lead to the...Ch. 11.12 - Prob. 22PCh. 11.12 - Prob. 23PCh. 11.13 - Prob. 24PCh. 11.13 - Prob. 25PCh. 11.14 - Prob. 26PCh. 11.14 - Prob. 27PCh. 11.14 - Prob. 28PCh. 11.15 - Prob. 29PCh. 11.15 - How would you synthesize the following compounds...Ch. 11 - Write a structure for each of the following a. N,N...Ch. 11 - Prob. 32PCh. 11 - Which ester is more reactive, methyl acetate or...Ch. 11 - What products would be formed from the reaction of...Ch. 11 - What products would be obtained from the following...Ch. 11 - Prob. 36PCh. 11 - a. Which compound would you expect to have a...Ch. 11 - a. List the following esters in order of...Ch. 11 - D. N. Kursanov, a Russian chemist, proved that the...Ch. 11 - Prob. 40PCh. 11 - Using an alcohol for one method and an alkyl...Ch. 11 - Prob. 42PCh. 11 - Prob. 44PCh. 11 - Prob. 45PCh. 11 - Prob. 46PCh. 11 - Prob. 47PCh. 11 - Prob. 48PCh. 11 - Prob. 49PCh. 11 - Show how the following compounds could be prepared...Ch. 11 - Prob. 51PCh. 11 - Prob. 52PCh. 11 - Prob. 53P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- During the synthesis of the antiasthmatic drug montelukast (Singulair), a silyl ether protecting group is used to mask the reactivity of an OH group. The silyl group chosen is the tert-butyldimethylsilyl (TBS) group. Draw the product of the following transformation, assuming the TBS-Cl reagent reacts only once with the starting material. Briefly explain your answer.arrow_forwardWhich of the following starting materials and reagents would be best to produce 2-hexanol? a. heptanal and 1. CH3MgBr 2. H3O* b. pentanal and 1. CH3MgBr, 2. H30* c. hexanal and 1. CH3MgBr, 2. H30* d. butanal and 1. CH3CH2MgBr, 2. H3O*arrow_forwardWhich alkyl halides were used in the acetoacetic ester synthesis to make the substituted ketone below? You may choose more than one answer. CH3BR CH3CH2CH2Br СНЗСН2Br O (CH3)2CHB.arrow_forward
- Please answer numbers 9 and 10 in the neatest way possible. Thanks!arrow_forwardIn the reaction of a nitrile with a Grignard reagent, there are two steps: a) nucleophilic addition of the Grignard to form an imine ion and b) hydrolysis of the imine to form a ketone. Draw the intermediate and the final product in this reaction. Ignore inorganic byproducts. 1. CH₂MgBr 2. H3O* Draw Imine Ion Intermediate 1. H30¹ 2. Neutralizing work-up aarrow_forward1. Br₂, PBrg 2. H₂O H₂C OH H3C OH Br The a-bromination of carbonyl compounds by Br₂ in acetic acid is limited to aldehydes and ketones because acids, esters, and amides don't enolize to a sufficient extent. Carboxylic acids, however, can be a-brominated by first converting the carboxylic acid to an acid bromide by treatment with PBr3. Following enolization of the acid bromide, Br₂ reacts in an α- substitution reaction. Hydrolysis of the acid bromide completes the reaction. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions H3C :0: :0::Br: Br Br H3C CO-P H Br Brarrow_forward
- 4. Write a complete mechanism for the base-catalyzed ester hydrolysis shown below. Why is the addition of acid required at the end of the reaction? OMe 1. KOH, H₂O, A 2. H₂O' OHarrow_forwardH3C OH 1. Br2, PBг3 2. H₂O H3C OH Br The a-bromination of carbonyl compounds by Br₂ in acetic acid is limited to aldehydes and ketones because acids, esters, and amides don't enolize to a sufficient extent. Carboxylic acids, however, can be a-brominated by first converting the carboxylic acid to an acid bromide by treatment with PBr3. Following enolization of the acid bromide, Br2 reacts in an a-substitution reaction. Hydrolysis of the acid bromide completes the reaction. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions Br Br зь P-Br Br H₂C H₂C SH Br Brarrow_forwarda. Rank the following esters from most reactive to least reactive in the first slow step of a nucleophilic acyl substitution reaction (formation of thetetrahedral intermediate): b. Rank the same esters from most reactive to least reactive in the second slow step of a nucleophilic acyl substitution reaction (collapse of thetetrahedralintermediate).arrow_forward
- 12 Why is ethanal a stronger acid than ethane? CH3-C-H ethanal CH3-CH3 ethane A. The conjugate base of ethanal (enolate ion) is stabilized by resonance; thus, the compulsion of ethanal to lose a H* (forming the enolate) is increased. Ethane's conjugate base is not stabilized. B. The inductive electron withdrawing effect of the C=O in ethanal increases its proclivity to release a H* C. Steric factors make ethanal the stronger acid. D. You can write resonance structures for ethanal (the carbon acid), indicating enhanced stability. Because the carbon acid is more stable, its acid strength is greater.arrow_forwardBelow is a structure of Levonorgestrel (Plan B®), used in prevention of pregancy. но HH H. Levonorgestrel Plan B® Below is one of the reactions used in the synthesis. What type of reaction is it? он :CEC-H Lc=c-H THF as solvent Levonorgestrel H,CO H,CO O A grignard reaction. Nucleophilic acyl substitution. O Nucleophilic addition. O An SN2 reaction. O Oarrow_forwardQ 6 pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License