
Loose Leaf for General, Organic and Biological Chemistry with Connect 2 Year Access Card
4th Edition
ISBN: 9781260269284
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Chapter 11.7, Problem 11.19P
Interpretation Introduction
Interpretation:
Whether vitamin
Concept introduction:
Water is a polar solvent. To dissolve in water, the compound must be polar. Polar compound is that compound in which polar bonds are present.The unequal sharing of valence electrons in a bond results in polar bond. Polar bond result when the bond formed between two atoms in which one atom is more electronegative than the other one. One example of polar bond is
Structure of HCl is as follows:
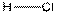
In
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Q1: Predict the major organic product(s) of the following reactions. Include stereochemistry
when necessary. Write NR if no reaction, try to explain.
1.) LDA, THF
2.)
СОН
CI
OH
H2SO4, heat
OH
m......
OH
1.) PCC, CH2Cl2
2.) CH3CH2MgBr, THF
3.) H3O+
4.) TsCl, pyr
5.) tBuOK, tBuOH
1.) SOCI 2, CHCI 3
2.) CH3CH2ONA, DMF
OH
1.) HBr
2.) Mg, THF
3.) H₂CO, THE
4.) H3O+
OH
NaH, THF
What is the stepwise mechanism for this reaction?
Draw the major product of this reaction
Chapter 11 Solutions
Loose Leaf for General, Organic and Biological Chemistry with Connect 2 Year Access Card
Ch. 11.1 - Prob. 11.1PCh. 11.2 - Fill in all H's and lone pairs in each compound.Ch. 11.3 - Prob. 11.2PPCh. 11.3 - Prob. 11.2PCh. 11.3 - Prob. 11.3PCh. 11.3 - Prob. 11.3PPCh. 11.3 - How many lone pairs are present in lidocaine, the...Ch. 11.4 - Convert each compound to a condensed formula.Ch. 11.4 - Convert each condensed formula to a complete...Ch. 11.4 - Convert each skeletal structure to a complete...
Ch. 11.4 - Prob. 11.5PCh. 11.4 - How many H’s are bonded to each indicated carbon...Ch. 11.4 - Using the skeletal structure, determine the...Ch. 11.5 - Prob. 11.7PCh. 11.5 - Prob. 11.8PCh. 11.5 - For each compound. [1] Identify the functional...Ch. 11.5 - How do a carboxylic acid and an alcohol differ?...Ch. 11.5 - Label each of the following condensed structures...Ch. 11.5 - Prob. 11.11PCh. 11.5 - Prob. 11.12PCh. 11.5 - Identify all of the functional groups in atenolol,...Ch. 11.5 - Prob. 11.13PCh. 11.5 - Prob. 11.10PPCh. 11.5 - Prob. 11.14PCh. 11.6 - Indicate the polar bonds in each compound. Label...Ch. 11.6 - Prob. 11.11PPCh. 11.6 - Prob. 11.16PCh. 11.6 - Predict the water solubility of each compound.Ch. 11.6 - Prob. 11.17PCh. 11.7 - Prob. 11.18PCh. 11.7 - Prob. 11.19PCh. 11.7 - Prob. 11.20PCh. 11 - Prob. 21PCh. 11 - Prob. 22PCh. 11 - Complete each structure by filling in all H’s and...Ch. 11 - Complete the structure of mepivacaine by filling...Ch. 11 - Prob. 25PCh. 11 - Prob. 26PCh. 11 - Prob. 27PCh. 11 - Prob. 28PCh. 11 - “Ecstasy” is a widely used illegal stimulant....Ch. 11 - Prob. 30PCh. 11 - Explain why each C—C—C bond angle in benzene...Ch. 11 - Prob. 32PCh. 11 - Convert each compound to a condensed structure.Ch. 11 - Convert each compound to a condensed structure.Ch. 11 - Convert each compound to a skeletal structure.Ch. 11 - Convert each compound to a skeletal structure.Ch. 11 - Convert each shorthand structure to a complete...Ch. 11 - Convert each shorthand structure to a complete...Ch. 11 - Convert each skeletal structure to a complete...Ch. 11 - Convert each skeletal structure to a complete...Ch. 11 - A and B are ball-and-stick models of two compounds...Ch. 11 - Prob. 42PCh. 11 - What is wrong in each of the following shorthand...Ch. 11 - Prob. 44PCh. 11 - Prob. 45PCh. 11 - Albuterol (trade names Proventil and Ventolin) is...Ch. 11 - Prob. 47PCh. 11 - Prob. 48PCh. 11 - Prob. 49PCh. 11 - (a) Identify the functional groups in donepezil,...Ch. 11 - Prob. 51PCh. 11 - GHB is an addictive, illegal recreational drug...Ch. 11 - Prob. 53PCh. 11 - Prob. 54PCh. 11 - Prob. 55PCh. 11 - Prob. 56PCh. 11 - Prob. 57PCh. 11 - (a) Identify the functional groups in venlafaxine,...Ch. 11 - You are given two unlabeled bottles of solids, one...Ch. 11 - State how potassium iodide (KI) and pentane...Ch. 11 - The given beaker contains 100 mL of the organic...Ch. 11 - Prob. 62PCh. 11 - Why do we need to know the shape of a molecule...Ch. 11 - 1,1-Dichloroethylene (CH2=CCl2) is a starting...Ch. 11 - Indicate the polar bonds in each molecule. Label...Ch. 11 - Indicate the polar bonds in each molecule. Label...Ch. 11 - Classify each molecule as polar or nonpolar.Ch. 11 - Classify each molecule as polar or nonpolar. a....Ch. 11 - Which molecule is more water soluble? Explain.Ch. 11 - Explain why pantothenic acid, vitamin B5, is water...Ch. 11 - Prob. 71PCh. 11 - Prob. 72PCh. 11 - Explain why regularly taking a large excess of a...Ch. 11 - You can obtain the minimum daily requirement of...Ch. 11 - Prob. 75PCh. 11 - Vitamin B6 is obtained by eating a diet that...Ch. 11 - Prob. 77PCh. 11 - Can an oxygen-containing organic compound, have...Ch. 11 - Prob. 79PCh. 11 - Prob. 80PCh. 11 - Benzocaine is the active ingredient in topical...Ch. 11 - Methyl salicylate is responsible for the...Ch. 11 - Answer the following questions about aldosterone,...Ch. 11 - Answer the following questions about...Ch. 11 - Prob. 85PCh. 11 - Skin moisturizers come in two types, (a) One type...Ch. 11 - THC is the active component in marijuana (Section...Ch. 11 - Cocaine is a widely abused, addicting drug....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please provide the IUPAC name for the compound shown herearrow_forwardProblem 6-29 Identify the functional groups in the following molecules, and show the polarity of each: (a) CH3CH2C=N CH, CH, COCH (c) CH3CCH2COCH3 NH2 (e) OCH3 (b) (d) O Problem 6-30 Identify the following reactions as additions, eliminations, substitutions, or rearrangements: (a) CH3CH2Br + NaCN CH3CH2CN ( + NaBr) Acid -OH (+ H2O) catalyst (b) + (c) Heat NO2 Light + 02N-NO2 (+ HNO2) (d)arrow_forwardPredict the organic product of Y that is formed in the reaction below, and draw the skeletal ("line") structures of the missing organic product. Please include all steps & drawings & explanations.arrow_forward
- Please choose the best reagents to complete the following reactionarrow_forwardProblem 6-17 Look at the following energy diagram: Energy Reaction progress (a) Is AG for the reaction positive or negative? Label it on the diagram. (b) How many steps are involved in the reaction? (c) How many transition states are there? Label them on the diagram. Problem 6-19 What is the difference between a transition state and an intermediate? Problem 6-21 Draw an energy diagram for a two-step reaction with Keq > 1. Label the overall AG°, transition states, and intermediate. Is AG° positive or negative? Problem 6-23 Draw an energy diagram for a reaction with Keq = 1. What is the value of AG° in this reaction?arrow_forwardProblem 6-37 Draw the different monochlorinated constitutional isomers you would obtain by the radical chlorination of the following compounds. (b) (c) Problem 6-39 Show the structure of the carbocation that would result when each of the following alkenes reacts with an acid, H+. (a) (b) (c)arrow_forward
- identify the carbonyl compound that is incapable of forming an enolate ionarrow_forwardpredict the product formed by the reaction of one mole each of cyclohex-2-en-1-one and lithium diethylcuprate. Assume a hydrolysis step follows the additionarrow_forwardPlease handwriting for questions 1 and 3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY