
ORGANIC CHEMISTRY-W/S.G+SOLN.MANUAL
8th Edition
ISBN: 9780134595450
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 11.5, Problem 26P
Interpretation Introduction
Interpretation:
The products obtained from metathesis of the following
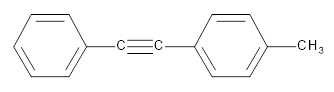
Concept introduction:
Alkene Metathesis Or olefin metathesis
This breaks the double bond of an alkene and then rejoins the fragments. When the fragments are joined, the new double bond is formed between two
Terminal alkene gives the best yields of a single alkene product in metathesis because one of the products is ethane, which is equally removed from the reaction mixture, thus shifting the equilibrium in favor of the other new alkene product.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1) Calculate the longest and shortest wavelengths in the Lyman and Paschen series.
2) Calculate the ionization energy of He* and L2+ ions in their ground states.
3) Calculate the kinetic energy of the electron emitted upon irradiation of a H-atom in ground state by a 50-nm radiation.
Calculate the ionization energy of He+ and Li²+ ions in their ground states.
Thannnxxxxx sirrr
Ahehehehehejh27278283-4;*; shebehebbw $+$;$-;$-28283773838 hahhehdva
Plleeaasseee solllveeee question 3 andd thankss sirr, don't solve it by AI plleeaasseee don't use AI
Chapter 11 Solutions
ORGANIC CHEMISTRY-W/S.G+SOLN.MANUAL
Ch. 11.1 - Prob. 1PCh. 11.2 - Which is more reactive an organolithium compound...Ch. 11.2 - Prob. 3PCh. 11.3 - Muscalure is the sex attractant of the common...Ch. 11.3 - Prob. 7PCh. 11.3 - Prob. 8PCh. 11.3 - Prob. 9PCh. 11.3 - Prob. 10PCh. 11.4 - Prob. 13PCh. 11.4 - Prob. 14P
Ch. 11.4 - Prob. 15PCh. 11.4 - Prob. 16PCh. 11.4 - Prob. 17PCh. 11.4 - Prob. 19PCh. 11.4 - Show how the Suzuki and/or Heck reactions can be...Ch. 11.4 - Identify two pairs of an alkyl bromide and an...Ch. 11.5 - Prob. 22PCh. 11.5 - Draw the product of ring-closing metathesis for...Ch. 11.5 - Prob. 25PCh. 11.5 - Prob. 26PCh. 11 - Prob. 27PCh. 11 - Prob. 28PCh. 11 - The coupling of an alkyne with an aryl halide in...Ch. 11 - Identify A through H.Ch. 11 - Using the given starting material, any necessary...Ch. 11 - What alkyl halide reacts with lithium...Ch. 11 - Prob. 33PCh. 11 - Prob. 34PCh. 11 - The following compound undergoes an intramolecular...Ch. 11 - Using ethynyleyclohexane as a starting material...Ch. 11 - Prob. 37PCh. 11 - Using the given starting material, any necessary...Ch. 11 - Prob. 39PCh. 11 - A student added an equivalent of...Ch. 11 - Using the given starting material, any necessary...Ch. 11 - Prob. 42PCh. 11 - Prob. 43PCh. 11 - Bombykol is the sex pheromone of the silk moth....Ch. 11 - Prob. 45PCh. 11 - Prob. 46PCh. 11 - A dibromide loses only one bromine when it reacts...Ch. 11 - What starting material is required in order to...Ch. 11 - What product is obtained from ring-opening...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the chemical shifts in 13C and 1H NMR for 4-chloropropiophenone ? Write structure and label hydrogens and carbonsarrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuu, don't solve it by AI plleeaasseeearrow_forward
- Please sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forward4. Read paragraph 4.15 from your textbook, use your calculated lattice energy values for CuO, CuCO3 and Cu(OH)2 an explain thermal decomposition reaction of malachite: Cu2CO3(OH)2 →2CuO + H2O + CO2 (3 points)arrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forward
- III O Organic Chemistry Using wedges and dashes in skeletal structures Draw a skeletal ("line") structure for each of the molecules below. Be sure your structures show the important difference between the molecules. key O O O O O CHON Cl jiii iiiiiiii You can drag the slider to rotate the molecules. Explanation Check Click and drag to start drawing a structure. Q Search X G ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use F 3 W C 3/5arrow_forward3. Use Kapustinskii's equation and data from Table 4.10 in your textbook to calculate lattice energies of Cu(OH)2 and CuCO3 (4 points)arrow_forward2. Copper (II) oxide crystalizes in monoclinic unit cell (included below; blue spheres 2+ represent Cu²+, red - O²-). Use Kapustinski's equation (4.5) to calculate lattice energy for CuO. You will need some data from Resource section of your textbook (p.901). (4 points) CuOarrow_forward
- What is the IUPAC name of the following compound? OH (2S, 4R)-4-chloropentan-2-ol O (2R, 4R)-4-chloropentan-2-ol O (2R, 4S)-4-chloropentan-2-ol O(2S, 4S)-4-chloropentan-2-olarrow_forwardIn the answer box, type the number of maximum stereoisomers possible for the following compound. A H H COH OH = H C Br H.C OH CHarrow_forwardSelect the major product of the following reaction. Br Br₂, light D Br Br Br Brarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning