
(a)
Interpretation:
The product of the given reaction has to be determined.
Concept introduction:
Suzuki reaction:
A reaction that couples an aryl halide or vinyl halide with an Organoboron reagent.
Heck reaction:
A reaction that breaks the double bond of an
Organoborane compound:
An alkyl–organoboron compound, an alkenyl–Organoboron compound, or an aryl–Organoboron compound:
Coupling reaction:
A reaction that joins two groups with a carbon-carbon bond.
(a)
Explanation of Solution
When a Iodobenzene is treated with alkene in presence of HeeK Reagent to form the correspondng the benzene product.
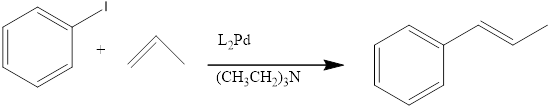
(b)
Interpretation:
The product of the given reaction has to be determined.
Concept introduction:
Suzuki reaction:
A reaction that couples an aryl halide or vinyl halide with an Organoboron reagent.
Heck reaction:
A reaction that breaks the double bond of an alkene and then joins the fragments.
Organoborane compound:
An alkyl–organoboron compound, an alkenyl–Organoboron compound, or an aryl–Organoboron compound:
Coupling reaction:
A reaction that joins two groups with a carbon-carbon bond.
(b)
Answer to Problem 27P
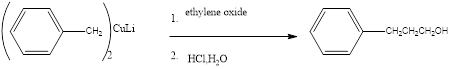
Explanation of Solution
When a Complex of benzene is treated with ethylene oxide to form the correspondng the alcoholic benzene product.
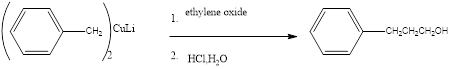
(c)
Interpretation:
The expected product should be formed by the following reaction.
Concept introduction:
Suzuki reaction:
A reaction that couples an aryl halide or vinyl halide with an Organoboron reagent.
Heck reaction:
A reaction that breaks the double bond of sa alkene and then joins the fragments.
Organoborane compound:
An alkyl –organoboron compound, an alkenyl –Organoboron compound, or an aryl –Organoboron compound:
Coupling reaction:
A reaction that joins two groups with a carbon-carbon bond.
(c)
Answer to Problem 27P

Explanation of Solution
Chloro benzene is treated with alkyl substituted CuLi reagent to give the corresponding product.

(d)
Interpretation:
The expected product should be formed by the following reaction.
Concept introduction:
Suzuki reaction:
A reaction that couples an aryl halide or vinyl halide with an Orghanoboron reagent.
Heck reaction:
A reaction that breaks the double bond of sa alkene and then joins the fragments.
Organoborane compound:
An alkyl –organoboron compound, an alkenyl –Organoboron compound, or an aryl –Organoboron compound:
Coupling reaction:
A reaction that joins two groups with a carbon-carbon bond.
(d)
Answer to Problem 27P
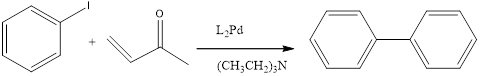
Explanation of Solution
When a Iodobenzene is treated with
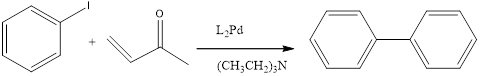
(e)
Interpretation:
The expected product should be formed by the following reaction.
Concept introduction:
Suzuki reaction:
A reaction that couples an aryl halide or vinyl halide with an Organoboron reagent.
Heck reaction:
A reaction that breaks the double bond of sa alkene and then joins the fragments.
Organoborane compound:
An alkyl – organoboron compound, an alkenyl –Organoboron compound, or an aryl –Organoboron compound:
Coupling reaction:
A reaction that joins two groups with a carbon-carbon bond.
(e)
Answer to Problem 27P
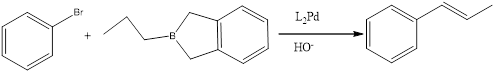
Explanation of Solution
When a bromo benzene treated with a brane complecx in presence of Heek reagent to give the corresponding product.

(f)
Interpretation:
The expected product should be formed by the following reaction.
Concept introduction:
Suzuki reaction:
A reaction that couples an aryl halide or vinyl halide with an Organoboron reagent.
Heck reaction:
A reaction that breaks the double bond of an alkene and then joins the fragments.
Organoborane compound:
An alkyl–organoboron compound, an alkenyl–Organoboron compound, or an aryl–Organoboron compound:
Coupling reaction:
A reaction that joins two groups with a carbon-carbon bond.
(f)
Answer to Problem 27P
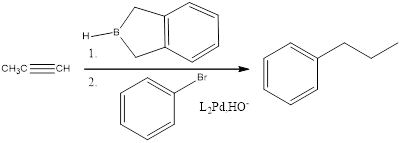
Explanation of Solution
When an

Want to see more full solutions like this?
Chapter 11 Solutions
ORGANIC CHEMISTRY-W/S.G+SOLN.MANUAL
- What alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. and two equivalents of CH2=O draw structure ...arrow_forwardH-Br Energy 1) Draw the step-by-step mechanism by which 3-methylbut-1-ene is converted into 2-bromo-2-methylbutane. 2) Sketch a reaction coordinate diagram that shows how the internal energy (Y- axis) of the reacting species change from reactants to intermediate(s) to product. Brarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 H-CI CH2Cl2 CIarrow_forward
- Draw the products of the stronger acid protonating the other reactant. དའི་སྐད”“ H3C OH H3C CH CH3 KEq Product acid Product basearrow_forwardDraw the products of the stronger acid protonating the other reactant. H3C NH2 NH2 KEq H3C-CH₂ 1. Product acid Product basearrow_forwardWhat alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. draw structure ... andarrow_forward
- Draw the products of the stronger acid protonating the other reactant. H3C-C=C-4 NH2 KEq CH H3C `CH3 Product acid Product basearrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 Br H-Br CH2Cl2 + enant.arrow_forwardDraw the products of the stronger acid protonating the other reactant. KEq H₂C-O-H H3C OH Product acid Product basearrow_forward
- Draw the products of the stronger acid protonating the other reactant. OH KEq CH H3C H3C `CH3 Product acid Product basearrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). Ph H-I CH2Cl2arrow_forward3 attempts left Check my work Draw the products formed in the following oxidative cleavage. [1] 03 [2] H₂O draw structure ... lower mass product draw structure ... higher mass productarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





