
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
12th Edition
ISBN: 9780321908445
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11.5, Problem 11.26QAP
Give the IUPAC name for each of the following:
a. H2C = CH— CH2— CH3
b.
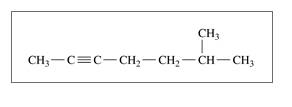
c.
d. CH3— CH2— CH = CH— CH3
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Part 2. A solution of 6.00g of substance B in 100.0mL of aqueous solution is in equilibrium, at room temperature,
wl a solution of B in diethyl ether (ethoxyethane) containing 25.0 g of B in 50.0 mL
9) what is the distribution coefficient of substance B
b) what is
the mass of B extracted by shaking 200 ml of an aqueous solution containing
10g of B with call at room temp):
i) 100 mL of
diethyl ether
ii) 50ml of diethyl ether twice
iii) 25ml of diethyl ether four times
- Rank the following groups of compounds from most acidic (1) to least acidic (4). Place the number
corresponding to the compound's relative rank in the blank below the structure.
a.
NO₂
NO₂
CH2CH2CH2CH2OH
CH3 CH3CH2CHOH
CH3CH2CH2CH2OH
NO₂
CH3CHCH2CH2OH
b.
OH
OH
CH₂OH
CO₂H
HC
CN
CN
CN
Give the major organic product(s) of the following reactions or sequences of reactions. Show all
relevant stereochemistry
a.
H
MgBr
1. ether
2. H₂O*
4
COH
b.
1. LIAIH, ether
2. H₂O
Choose the best reagent(s) for carrying out the following conversions from the list provided below.
Place the letter of the best choice in the blank to the left of the conversion. Reagents may be used
more than once.
a. 1.
CH3MgBr, ether
2. H3O+
NaOH
b. 1.
PBr3
2.
C.
2.
1. (CH3)3SiCl, (CH3CH2)3N
CH3MgBr, ether
3. H₂O*+
2. H3O+
e. 1. p-TosCl, pyridine
f.
نها
g.
2. NaOH
CrO3, H₂SO4, H₂O
1.
NaBH4, ethanol
2. H30*
h. PCC, CH2Cl2
Ovoldo-6
a.
b.
OH
OH
H
OH
O
any organic
Chapter 11 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Ch. 11.1 - Prob. 11.1QAPCh. 11.1 - Prob. 11.2QAPCh. 11.1 - Identify each of the following properties as more...Ch. 11.1 - Identify each of the following properties as more...Ch. 11.1 - Prob. 11.5QAPCh. 11.1 - Prob. 11.6QAPCh. 11.2 - Give the IUPAC name for each of the following...Ch. 11.2 - Give the IUPAC name for each of the following...Ch. 11.2 - Prob. 11.9QAPCh. 11.2 - Draw the condensed structural formula for alkanes...
Ch. 11.3 - Indicate whether each of the following pairs...Ch. 11.3 - Indicate whether each of the following pairs...Ch. 11.3 - Give the IUPAC name for each of the following a....Ch. 11.3 - Give the TUPAC name for each of the following: a....Ch. 11.3 - Draw the condensed structural formula for each of...Ch. 11.3 - Draw the condensed structural formula for each of...Ch. 11.3 - Draw the line-angle formula for each of the...Ch. 11.3 - Prob. 11.18QAPCh. 11.4 - Heptane, used as a solvent for rubber cement, has...Ch. 11.4 - Nonane has a density of 0.79 g/mL and boils at 151...Ch. 11.4 - Prob. 11.21QAPCh. 11.4 - Prob. 11.22QAPCh. 11.5 - Prob. 11.23QAPCh. 11.5 - Identify the following as alkanes, alkenes,...Ch. 11.5 - Give the IUPAC name for each of the following: a....Ch. 11.5 - Give the IUPAC name for each of the following: a....Ch. 11.5 - Draw the condensed structural formula, or...Ch. 11.5 - Prob. 11.28QAPCh. 11.6 - Prob. 11.29QAPCh. 11.6 - Prob. 11.30QAPCh. 11.6 - Prob. 11.31QAPCh. 11.6 - Prob. 11.32QAPCh. 11.7 - Prob. 11.33QAPCh. 11.7 - Prob. 11.34QAPCh. 11.8 - Prob. 11.35QAPCh. 11.8 - Prob. 11.36QAPCh. 11.8 - Prob. 11.37QAPCh. 11.8 - Prob. 11.38QAPCh. 11 - Prob. 11.39UTCCh. 11 - Prob. 11.40UTCCh. 11 - Prob. 11.41UTCCh. 11 - Prob. 11.42UTCCh. 11 - Prob. 11.43UTCCh. 11 - Convert each of the following line-angle formulas...Ch. 11 - Give the IUPAC name for each of the following:...Ch. 11 - Give the IUPAC name for each of the following:...Ch. 11 - Give the IUPAC name (including cis or trans, if...Ch. 11 - Give the LUPAC name (including cis or trans, if...Ch. 11 - Prob. 11.49AQAPCh. 11 - Prob. 11.50AQAPCh. 11 - Prob. 11.51AQAPCh. 11 - Prob. 11.52AQAPCh. 11 - Draw the condensed structural or line-angle...Ch. 11 - Draw the condensed structural or line-angle...Ch. 11 - Draw the cis and trans isomers for each of the...Ch. 11 - Draw the cis and trans isomers for each of the...Ch. 11 - Prob. 11.57AQAPCh. 11 - Prob. 11.58AQAPCh. 11 - Prob. 11.59AQAPCh. 11 - Prob. 11.60AQAPCh. 11 - Prob. 11.61AQAPCh. 11 - Give the name for the product from the...Ch. 11 - Prob. 11.63AQAPCh. 11 - Prob. 11.64AQAPCh. 11 - Prob. 11.65CQCh. 11 - Prob. 11.66CQCh. 11 - Prob. 11.67CQCh. 11 - Prob. 11.68CQCh. 11 - Prob. 11.69CQCh. 11 - Prob. 11.70CQCh. 11 - Prob. 11.71CQCh. 11 - Margarines are produced from the hydrogenation of...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine the rate law for sodium thiosulfate from the following data: [Na2S2O3] Time (s) 0.0318 230. 0.0636 57.5arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardDetermine the rate order from the following data: [Na2S2O3] Time (s) 0.0455 M 180.0 0.0909 M 90.0 0.136 M 60.0 Group of answer choices First order Second order Third order Zero orderarrow_forward
- Write down by equation of the following reactions: the Clemmensen reduction and synthesize of a secondary alcarrow_forwardPropose syntheses of the following compounds starting with benzene or toluene. Assume ortho and isomers can be separated. a. b. O₂N- Cl COOH para 0. Propose syntheses to carry out each of the following conversions. Assume ortho and para isomers can be separated a. Br b. COOH CH3 NH₂ PABA (active ingredient in some sunscreens)arrow_forwardH3C H C=C CH3 H m-chloroperoxybenzoic acid CH2Cl2, rt C-C--. H3CH2CC H H3C CH3 Cl₂ H₂O NaOH H₂O D. S- E. CH3 H₂O₂, H₂O It CH₂O Na + CHI F. HI, H₂O heat G. 4 OH CH3CHCH3 + ICH2CH3 1. NaH (CH3)3COH (CH3)3 COCHCH2CH3 2. CH3arrow_forward
- 5. Show how the ether below could be prepared from toluene and any other necessary reagents. Show all reagents and all intermediate structures. H3C- H3C- CI OCH2CH3arrow_forwardGiven the major organic product(s) of each of the following reactions. If none is predicted, write "N.R." [answer 61 a. b. H3C C. NO₂ CH3CH2CH2Cl AICI 3 1) NaOH CI 2) H3O+ NO₂ 1. SnCl2, H3O+ 2. NaOH 3arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- To answer the following questions, consider the reaction below: CH3 . CH3 OH a. The best reagents for accomplishing the above transformation are.... a. 1. OsO4, pyridine 2. NaHSO3, H₂O b. 1. Hg(OAc)2, H₂O 1. C. 2. NaBH4 RCO₂H, CH2Cl₂ 2. H₂O* d. 1. BH3, THF 2. H₂O₂, OH b. The alcohol product is classified as a: a. 1° alcohol b. 2° alcohol C. 3° alcohol d. 4° alcohol c. The conversion of an alcohol into an alkyl chloride by reaction with SOCI2 is an example of: a. b. ن نخنه C. d. an El process an Syl process an E2 process an Sy2 processarrow_forwardEstimation of ash in food Questions: Q1: What does the word ash refer to? Q2: Mention the types of ash in food Q3: Mention the benefit of using a glass dryerarrow_forwardDraw structures corresponding to the names given a. m-fluoronitrobenzene b. p-bromoaniline c. o-chlorophenol d. 3,5-dimethylbenzoic acidarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY