
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
12th Edition
ISBN: 9780321908445
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11.5, Problem 11.24QAP
Identify the following as
a.
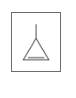
b.
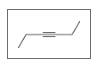
c.
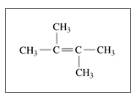
d.
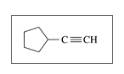
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please correct answer and don't use hand rating
Don't used hand raiting
Reagan is doing an atomic absorption experiment that requires a set of zinc standards in the 0.4-
1.6 ppm range. A 1000 ppm Zn solution was prepared by dissolving the necessary amount of
solid Zn(NO3)2 in water. The standards can be prepared by diluting the 1000 ppm Zn solution.
Table 1 shows one possible set of serial dilutions (stepwise dilution of a solution) that Reagan
could perform to make the necessary standards. Solution A was prepared by diluting 5.00 ml of
the 1000 ppm Zn standard to 50.00 ml. Solutions C-E are called "calibration standards" because
they will be used to calibrate the atomic absorption spectrometer.
Table 1: Dilutions of Zinc Solutions
Solution
Zinc Solution
Volume
Diluted Solution
Concentration
used
volume
(ppm Zn)
(mL)
(mL)
concentration
(ppm Zn)
Solution
concentration
A
1000
5.00
50.00
1.00×10²
(ppm
Zn(NO3)2)
2.90×10²
Solution
concentration
(M Zn(NO3)2
1.53×10-3
B
Solution A 5.00
100.00
5.00
C
Solution B 5.00 50.00
0.50
7.65×10-6
D
Solution B 10.00 50.00…
Chapter 11 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Ch. 11.1 - Prob. 11.1QAPCh. 11.1 - Prob. 11.2QAPCh. 11.1 - Identify each of the following properties as more...Ch. 11.1 - Identify each of the following properties as more...Ch. 11.1 - Prob. 11.5QAPCh. 11.1 - Prob. 11.6QAPCh. 11.2 - Give the IUPAC name for each of the following...Ch. 11.2 - Give the IUPAC name for each of the following...Ch. 11.2 - Prob. 11.9QAPCh. 11.2 - Draw the condensed structural formula for alkanes...
Ch. 11.3 - Indicate whether each of the following pairs...Ch. 11.3 - Indicate whether each of the following pairs...Ch. 11.3 - Give the IUPAC name for each of the following a....Ch. 11.3 - Give the TUPAC name for each of the following: a....Ch. 11.3 - Draw the condensed structural formula for each of...Ch. 11.3 - Draw the condensed structural formula for each of...Ch. 11.3 - Draw the line-angle formula for each of the...Ch. 11.3 - Prob. 11.18QAPCh. 11.4 - Heptane, used as a solvent for rubber cement, has...Ch. 11.4 - Nonane has a density of 0.79 g/mL and boils at 151...Ch. 11.4 - Prob. 11.21QAPCh. 11.4 - Prob. 11.22QAPCh. 11.5 - Prob. 11.23QAPCh. 11.5 - Identify the following as alkanes, alkenes,...Ch. 11.5 - Give the IUPAC name for each of the following: a....Ch. 11.5 - Give the IUPAC name for each of the following: a....Ch. 11.5 - Draw the condensed structural formula, or...Ch. 11.5 - Prob. 11.28QAPCh. 11.6 - Prob. 11.29QAPCh. 11.6 - Prob. 11.30QAPCh. 11.6 - Prob. 11.31QAPCh. 11.6 - Prob. 11.32QAPCh. 11.7 - Prob. 11.33QAPCh. 11.7 - Prob. 11.34QAPCh. 11.8 - Prob. 11.35QAPCh. 11.8 - Prob. 11.36QAPCh. 11.8 - Prob. 11.37QAPCh. 11.8 - Prob. 11.38QAPCh. 11 - Prob. 11.39UTCCh. 11 - Prob. 11.40UTCCh. 11 - Prob. 11.41UTCCh. 11 - Prob. 11.42UTCCh. 11 - Prob. 11.43UTCCh. 11 - Convert each of the following line-angle formulas...Ch. 11 - Give the IUPAC name for each of the following:...Ch. 11 - Give the IUPAC name for each of the following:...Ch. 11 - Give the IUPAC name (including cis or trans, if...Ch. 11 - Give the LUPAC name (including cis or trans, if...Ch. 11 - Prob. 11.49AQAPCh. 11 - Prob. 11.50AQAPCh. 11 - Prob. 11.51AQAPCh. 11 - Prob. 11.52AQAPCh. 11 - Draw the condensed structural or line-angle...Ch. 11 - Draw the condensed structural or line-angle...Ch. 11 - Draw the cis and trans isomers for each of the...Ch. 11 - Draw the cis and trans isomers for each of the...Ch. 11 - Prob. 11.57AQAPCh. 11 - Prob. 11.58AQAPCh. 11 - Prob. 11.59AQAPCh. 11 - Prob. 11.60AQAPCh. 11 - Prob. 11.61AQAPCh. 11 - Give the name for the product from the...Ch. 11 - Prob. 11.63AQAPCh. 11 - Prob. 11.64AQAPCh. 11 - Prob. 11.65CQCh. 11 - Prob. 11.66CQCh. 11 - Prob. 11.67CQCh. 11 - Prob. 11.68CQCh. 11 - Prob. 11.69CQCh. 11 - Prob. 11.70CQCh. 11 - Prob. 11.71CQCh. 11 - Margarines are produced from the hydrogenation of...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (b) Provide the number of peaks in each of the indicated signals ('H NMR) for the compound below. CH3 6 1 H&C. C H₂ H2 3 HA 2 2 4 5 5arrow_forward8. The emission spectrum below for a one-electron (hydrogen-like) species in the gas phase shows all the lines, before they merge together, resulting from transitions to the ground state from higher energy states. Line A has a wavelength of 10.8 nm. BA Increasing wavelength, \ - a) What are the upper and lower principal quantum numbers corresponding to the lines labeled A and B? b) Identify the one-electron species that exhibits the spectrum.arrow_forwardShow work with explanation....don't give Ai generated solutionarrow_forward
- achieve.macmillanlearning.com Canvas EA eac h Hulu YouTube G 3 methyl cyclobutanol - Google Search Ranking Phenol Acidity Course -236 - Organic Chemistry - Mac... ← Assessment Completed 10 of 22 Questions 1 + Netflix paramount plus chem hw Galdehyde reaction with grignard reagent... b My Questions | bartleby M Inbox - chenteislegit@gmail.com - Gmail Due: Fri, Jan 31 Resources Solution Penalized ? Hint Submit Answer Use retrosynthetic analysis to suggest two paths to synthesize 2-methyl-3-hexanol using the Grignard reaction. (Click and drag the appropriate image to the correct position in the reactions.) Route 1 Aldehyde 1 or +98 Aldehyde 2 Route 2 Q6 +100 Solved in 1 attempt Q7 +95 Solved in 2 attempts Q8 +98 Unlimited attempts possible + + Grignard 1 OH H3O+ Grignard 2 Answer Bank Q9 +90 MgBr Unlimited attempts possible CH3CH2CH2MgBr Q10 Unlimited attempts Q11 ? ? +100 in 1 attempt 2-methyl-3-hexanol CH3CH2MgBr H H о H Attempt 3arrow_forward2) (4 pt) After the reaction was completed, the student collected the following data. Crude product data is the data collected after the reaction is finished, but before the product is purified. "Pure" product data is the data collected after attempted purification using recrystallization. Student B's data: Crude product data "Pure" product data after recrystallization Crude mass: 0.93 g grey solid Crude mp: 96-106 °C Crude % yield: Pure mass: 0.39 g white solid Pure mp: 111-113 °C Pure % yield: a) Calculate the crude and pure percent yields for the student's reaction. b) Summarize what is indicated by the crude and pure melting points.arrow_forwardDon't used hand raitingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning  World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License