
(a)
The mass flow rate of the refrigerant through the upper compression cycle.
(a)
Answer to Problem 59P
The mass flow rate of the refrigerant through the upper compression cycle is
Explanation of Solution
Sketch the schematic diagram for the two stage cascade refrigeration system as in Figure (1).
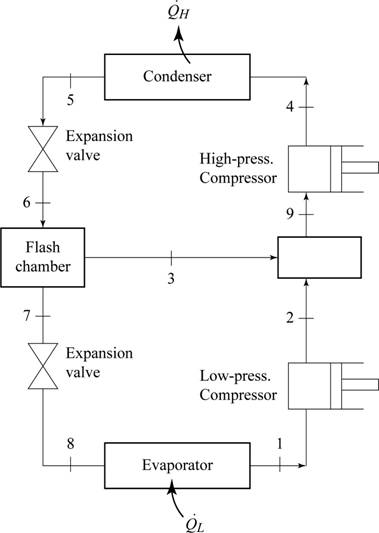
Write the relation between the specific enthalpies at the inlet and exit of throttling process.
Here, specific enthalpy at the inlet of throttling is
Write the expression for the isentropic efficiency of the compressor
Here, specific enthalpy at the isentropic exit of compressor is
Write the formula to calculate the dryness fraction at the exit of expansion valve
Here, specific enthalpy of refrigerant at expansion valve exit is
Write the expression for the mass flow rate of refrigerant
Here, mass flow rate of refrigerant at the inlet of low pressure compressor is
Conclusion:
From the Table A-12 of “Saturated refrigerant R-134a: Pressure”, obtain the properties of refrigerant at low pressure compressor inlet pressure
Here, specific enthalpy of the saturated vapor is
The specific entropy at the end of isentropic compression
Refer to Table A-13, “Superheated R-134a”, and obtain the values of R-134a at pressure of
Substitute
From the Table A-12 of “Saturated refrigerant R-134a: Pressure”, obtain the properties of refrigerant at throttling inlet pressure
From the Table A-12 of “Saturated refrigerant R-134a: Pressure”, obtain the properties of refrigerant at second stage compressor inlet pressure
Substitute
From the Table A-12 of “Saturated refrigerant R-134a: Pressure”, obtain the properties of refrigerant at second stage expansion inlet pressure
Substitute
From the Table A-12 of “Saturated refrigerant R-134a: Pressure”, obtain the properties of refrigerant at pressure
Substitute
Substitute
The mass flow rate of the refrigerant through the upper compression cycle is
(b)
The rate at which heat removed from the refrigerated space.
(b)
Answer to Problem 59P
The rate at which heat removed from the refrigerated space is
Explanation of Solution
Write the mass balance equation for the
Write the energy balance equation for the flash chamber.
Here, specific enthalpy at state 9 is
Write the formula to calculate the rate of heat transfer from the refrigerated space
Conclusion:
Refer to Table A-13, “Superheated R-134a”, and obtain the values of R-134a at pressure of
The specific entropy at the end of isentropic compression
Refer to Table A-13, “Superheated R-134a”, and obtain the values of R-134a at pressure of
Substitute
Substitute
Thus, the rate at which heat removed from the refrigerated space is
(c)
The power input required to the two stage cascade refrigeration system.
The coefficient of refrigeration for the two-stage cascade refrigeration system.
(c)
Answer to Problem 59P
The power input required to the two stage cascade refrigeration system is
The coefficient of refrigeration for the two-stage cascade refrigeration system is
Explanation of Solution
Write the formula to calculate the total required work input
Here, required work input to the first stage compression is
Write the formula to calculate the COP of the two-stage cascade refrigeration system.
Conclusion:
Substitute
Thus, the power input required to two stage cascade refrigeration system is
Substitute 26.35 kW for
Thus, the coefficient of refrigeration for the two-stage cascade refrigeration system is
(d)
The rate at which heat removed from the refrigerated space.
The coefficient of refrigeration for the two-stage cascade refrigeration system.
(d)
Answer to Problem 59P
The rate at which heat removed from the refrigerated space is
The coefficient of refrigeration for the two-stage cascade refrigeration system is
Explanation of Solution
Write the formula to calculate the rate of heat transfer from the refrigerated space
Here, the specific enthalpy of refrigerant at the exit of expansion valve is
Write the formula to calculate the required work input
Conclusion:
From the Table A-12 of “Saturated refrigerant R-134a: Pressure”, obtain the properties of refrigerant at low pressure compressor inlet pressure
Here, specific enthalpy of the saturated vapor is
The specific entropy at the end of isentropic compression
Refer to Table A-13, “Superheated R-134a”, and obtain the values of R-134a at pressure of
Substitute
From the Table A-12 of “Saturated refrigerant R-134a: Pressure”, obtain the properties of refrigerant at expansion valve inlet pressure
Substitute
Substitute
Thus, the rate at which heat removed from the refrigerated space is
Substitute
Substitute 25.67 kW for
Thus, the coefficient of refrigeration for the two-stage cascade refrigeration system is
Want to see more full solutions like this?
Chapter 11 Solutions
EBK THERMODYNAMICS: AN ENGINEERING APPR
- A heat transfer experiment is conducted on two identical spheres which are initially at the same temperature. The spheres are cooled by placing them in a channel. The fluid velocity in the channel is non-uniform, having a profile as shown. Which sphere cools off more rapidly? Explain. V 1arrow_forwardMy ID# 016948724 last 2 ID# 24 Last 3 ID# 724 Please help to find the correct answer for this problem using my ID# first write le line of action and then help me to find the forces {fx= , fy= mz= and for the last find the moment of inertial about the show x and y axes please show how to solve step by steparrow_forwardMy ID# 016948724 last 2 ID# 24 Last 3 ID# 724 Please help to find the correct answer for this problem using my ID# first write le line of action and then help me to find the forces and the tension {fx= , fy= mz=arrow_forward
- My ID# 016948724 last 2 ID# 24 Last 3 ID# 724 Please help to find the correct answer for this problem using my ID# first write le line of action and then help me to find the forces {fx= , fy= mz=arrow_forwardmy ID is 016948724 Last 2 ID# 24 Last 3 ID# 724 please help me to solve this problem step by step show me how to solve first wirte the line actions and then find the forces {fx=, fy=, mz= and for the last step find the support reactions and find forcesarrow_forwardUppgift 1 (9p) 3 m 3 m 3 m 3 m H G F 3 m ↑ Dy D B AAY 30° 8 kN Ay Fackverket i figuren ovan är belastat med en punktlast. Bestäm normalkraften i stängerna BC, BG och FG.arrow_forward
- The cardiovascular countercurrent heat exchnager mechanism is to warm venous blood from 28 degrees C to 35 degrees C at a mass flow rate of 2 g/s. The artery inflow temp is 37 degrees C at a mass flow rate of 5 g/s. The average diameter of the vein is 5 cm and the overall heat transfer coefficient is 125 W/m^2*K. Determine the overall blood vessel length needed too warm the venous blood to 35 degrees C if the specific heat of both arterial and venous blood is constant and equal to 3475 J/kg*K.arrow_forwardThe forces Qy=12 kNQy=12kN and Qz=16 kNQz=16kN act on the profile at the shear center C. Calculate: a) Shear flow at point B (2 points)b) Shear stress at point D (3 points)arrow_forwardConsider the feedback controlled blending system shown below, which is designed to keep theoutlet concentration constant despite potential variations in the stream 1 composition. The density of all streamsis 920 kg/m3. At the nominal steady state, the flow rates of streams 1 and 2 are 950 and 425 kg/min,respectively, the liquid level in the tank is 1.3 m, the incoming mass fractions are x1 = 0.27, x2 = 0.54. Noticethe overflow line, indicating that the liquid level remains constant (i.e. any change in total inlet flow ratetranslates immediately to the same change in the outlet flow rate). You may assume the stream 1 flowrate andthe stream 2 composition are both constant. Use minutes as the time unit throughout this problem. d) Derive the first order process and disturbance transfer functions;Gp= Kp/(tou*s+1) and Gd=Kd/(tou*s+1) and calculate and list the values and units of the parameters. e) Using the given information, write the general forms of Gm, GIP, and Gv below(in terms of…arrow_forward
- a) Briefly explain what ratio control is. Give an example of a common chemical engineering situation in whichratio control would be useful and for that example state exactly how ratio control works (what would bemeasured, what is set, and how the controller logic works).b) Briefly explain what cascade control is. Give an example of a common chemical engineering situation inwhich cascade control would be useful and for that example state exactly how cascade control works (whatwould be measured, what is set, and how the controller logic works).arrow_forwardDetermine the reaction force acting on the beam AB, given F = 680 N. 5 4 4 m 3 3 A B 30° 3 m F (N)arrow_forwardThe frame in the figure is made of an HEA 300 profile (E = 210 GPa, material S355).a) Determine the support reactions at point A. (1p)b) Sketch the bending moment diagram caused by the loading. (1p)c) Using the principle of virtual work (unit load method), calculate the vertical displacement at point B using moment diagrams. Also take into account the compression of the column. (3p)arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





