
Concept explainers
(a)
Interpretation:
The full electron configuration for the element with given
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electron configuration. The description of every electron in an orbital is given by the electron configuration of that atom.
Answer to Problem 96AP
The electron configuration of the given element with
Explanation of Solution
The electron configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is,
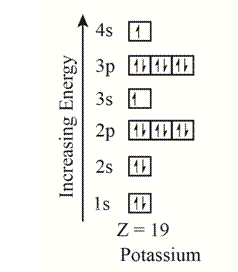
Figure 1
The electron configuration of the given element can also be written as
(b)
Interpretation:
The full electron configuration for the element with given atomic number, the orbital box diagram and the noble gas shorthand configuration ions are to be stated.
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electron configuration. The description of every electron that is moving freely in an orbital is given by the electron configuration of that atom.
Answer to Problem 96AP
The electron configuration of the given element with
Explanation of Solution
The electron configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is,
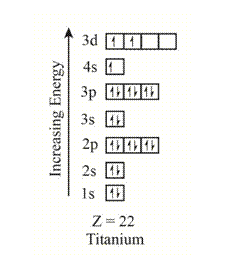
Figure 2
The electron configuration of the given element can also be written as
(c)
Interpretation:
The full electron configuration for the element with given atomic number, the orbital box diagram and the noble gas shorthand configuration ions are to be stated.
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electron configuration. The description of every electron in an orbital is given by the electron configuration of that atom.
Answer to Problem 96AP
The electron configuration of the given element with
Explanation of Solution
The electron configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is,
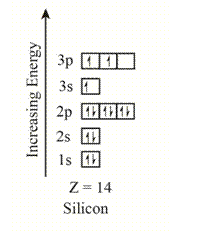
Figure 3
The electron configuration of the given element can also be written as
(d)
Interpretation:
The full electron configuration for the element with given atomic number, the orbital box diagram and the noble gas shorthand configuration ions are to be stated.
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electron configuration. The description of every electron in an orbital is given by the electron configuration of that atom.
Answer to Problem 96AP
The electron configuration of the given element with
Explanation of Solution
The electron configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is,
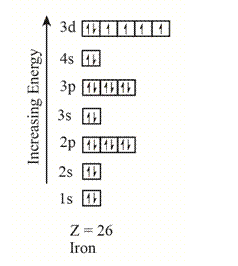
Figure 4
The electron configuration of the given element can also be written as
(e)
Interpretation:
The full electron configuration for the element with given atomic number, the orbital box diagram and the noble gas shorthand configuration ions are to be stated.
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electron configuration. The description of every electron in an orbital is given by the electron configuration of that atom.
Answer to Problem 96AP
The electron configuration of the given element with
Explanation of Solution
The electron configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is,
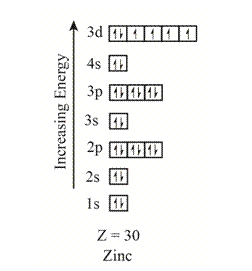
Figure 5
The electron configuration of the given element can also be written as
Want to see more full solutions like this?
Chapter 11 Solutions
Introductory Chemistry: Foundation - Text (Looseleaf)
- Would the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forwardPlease help me answer a. Please and thank you I advance.arrow_forwardDraw both of the chair flips for both the cis and trans isomers for the following compounds: 1,4-diethylcyclohexane 1-methyl-3-secbutylcyclohexanearrow_forward
- Ppplllleeeaaasssseeee hellppp wiithhh thisss physical chemistryyyyy I talked like this because AI is very annoyingarrow_forwardFor this question, if the product is racemic, input both enantiomers in the same Marvin editor. A) Input the number that corresponds to the reagent which when added to (E)-but-2-ene will result in a racemic product. Input 1 for Cl, in the cold and dark Input 2 for Oy followed by H₂O, Zn Input 3 for D₂ with metal catalyst Input 4 for H₂ with metal catalyst B) Draw the skeletal structure of the major organic product made from the reagent in part A Marvin JS Help Edit drawing C) Draw the skeletal structure of the major organic product formed when (2)-but-2-ene is treated with peroxyacetic acid. Marvin 35 Helparrow_forwardMichael Reactions 19.52 Draw the products from the following Michael addition reactions. 1. H&C CH (a) i 2. H₂O* (b) OEt (c) EtO H₂NEt (d) ΕΙΟ + 1. NaOEt 2. H₂O' H H 1. NaOEt 2. H₂O*arrow_forward
- Rank the labeled protons (Ha-Hd) in order of increasing acidity, starting with the least acidic. НОН НЬ OHd Онсarrow_forwardCan the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? ? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C :0 T Add/Remove step Garrow_forwardThe following equations represent the formation of compound MX. What is the AH for the electron affinity of X (g)? X₂ (g) → 2X (g) M (s) → M (g) M (g) M (g) + e- AH = 60 kJ/mol AH = 22 kJ/mol X (g) + e-X (g) M* (g) +X (g) → MX (s) AH = 118 kJ/mol AH = ? AH = -190 kJ/mol AH = -100 kJ/mol a) -80 kJ b) -30 kJ c) -20 kJ d) 20 kJ e) 156 kJarrow_forward
- A covalent bond is the result of the a) b) c) d) e) overlap of two half-filled s orbitals overlap of a half-filled s orbital and a half-filled p orbital overlap of two half-filled p orbitals along their axes parallel overlap of two half-filled parallel p orbitals all of the abovearrow_forwardCan the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C T Add/Remove step X ноarrow_forwardWhich one of the following atoms should have the largest electron affinity? a) b) c) d) 으으 e) 1s² 2s² 2p6 3s¹ 1s² 2s² 2p5 1s² 2s² 2p 3s² 3p² 1s² 2s 2p 3s² 3p6 4s2 3ds 1s² 2s² 2p6arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





