
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11, Problem 8PS
Which of the following compounds would be expected to form intermolecular hydrogen bonds in the liquid state?
- (a) H2Se
- (b) HCO2H (formic acid)
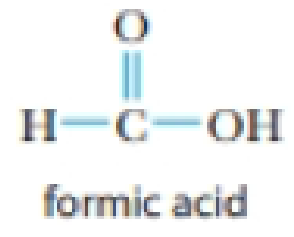
- (c) HI
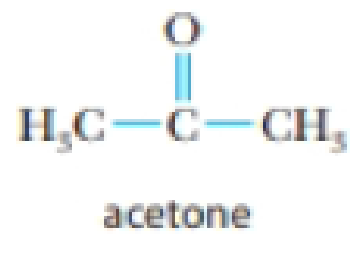
- (d) acetone, (CH3)2CO
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Write the amididation reaction mechanism of a-aminophenol and acetic acid to produce acetaminophen
For the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. 
How to draw the reaction mechasnism below
Chapter 11 Solutions
Chemistry & Chemical Reactivity
Ch. 11.2 - Which should have the more negative hydration...Ch. 11.3 - Using structural formulas, describe the hydrogen...Ch. 11.4 - Prob. 11.3CYUCh. 11.5 - Prob. 11.4CYUCh. 11.6 - The molar enthalpy of vaporization of methanol,...Ch. 11.6 - Prob. 11.6CYUCh. 11.6 - Prob. 1.1ACPCh. 11.6 - Prob. 1.2ACPCh. 11.6 - Prob. 2.1ACPCh. 11.6 - Prob. 2.2ACP
Ch. 11 - Prob. 1PSCh. 11 - Intermolecular forces: What type of forces must be...Ch. 11 - Prob. 3PSCh. 11 - Prob. 4PSCh. 11 - Considering intermolecular forces in the pure...Ch. 11 - Considering intermolecular forces in the pure...Ch. 11 - Prob. 7PSCh. 11 - Which of the following compounds would be expected...Ch. 11 - Prob. 9PSCh. 11 - When salts of Mg2+, Na+, and Cs+ are placed in...Ch. 11 - Prob. 11PSCh. 11 - The enthalpy of vaporization of liquid mercury is...Ch. 11 - Answer the following questions using Figure 11.12:...Ch. 11 - Answer the following questions using Figure 11.12:...Ch. 11 - Prob. 15PSCh. 11 - Refer to Figure 11.12 to answer these questions:...Ch. 11 - Which member of each of the following pairs of...Ch. 11 - Place the following four compounds in order of...Ch. 11 - Prob. 19PSCh. 11 - You are comparing three different substances, A,...Ch. 11 - Equilibrium vapor pressures of benzene, C6H6, at...Ch. 11 - Prob. 22PSCh. 11 - Can carbon monoxide (Tc = 132.9 K; Pc = 34.5 atm...Ch. 11 - Methane (CH4) cannot be liquefied at room...Ch. 11 - What is surface tension? Give an example...Ch. 11 - What factors affect the viscosity of a substance?...Ch. 11 - If a piece of filter paper (an absorbent paper...Ch. 11 - When water is placed in a buret it forms a concave...Ch. 11 - Prob. 29GQCh. 11 - What types of intermolecular forces are important...Ch. 11 - Which of the following salts, Li2SO4 or Cs2SO4, is...Ch. 11 - Prob. 32GQCh. 11 - Prob. 33GQCh. 11 - Prob. 34GQCh. 11 - Rank the following compounds in order of...Ch. 11 - Prob. 36GQCh. 11 - Prob. 37GQCh. 11 - The following data are the equilibrium vapor...Ch. 11 - Prob. 39ILCh. 11 - A hand boiler can be purchased in toy stores or at...Ch. 11 - Prob. 41ILCh. 11 - Prob. 42ILCh. 11 - Acetone, CH3COCH3, is a common laboratory solvent....Ch. 11 - Cooking oil floats on top of water. From this...Ch. 11 - Liquid ethylene glycol, HOCH2CH2OH, is one of the...Ch. 11 - Liquid methanol, CH3OH, is placed in a glass tube....Ch. 11 - Account for these facts: (a) Although ethanol...Ch. 11 - Prob. 48SCQCh. 11 - Prob. 49SCQCh. 11 - Prob. 50SCQCh. 11 - Prob. 51SCQCh. 11 - Prob. 52SCQCh. 11 - A fluorocarbon, CF4, has a critical temperature of...Ch. 11 - Prob. 55SCQCh. 11 - List four properties of liquids that are directly...Ch. 11 - List the following ions in order of hydration...Ch. 11 - Prob. 59SCQCh. 11 - An 8.82-g sample of Br2 is placed in an evacuated...Ch. 11 - Polarizability is defined as the extent to which...Ch. 11 - Prob. 62SCQCh. 11 - A pressure cooker (a kitchen appliance) is a pot...Ch. 11 - Vapor pressures of NH3() at several temperatures...Ch. 11 - Prob. 65SCQCh. 11 - Prob. 66SCQCh. 11 - Prob. 67SCQ
Additional Science Textbook Solutions
Find more solutions based on key concepts
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
2. Why is it that the range of resting blood pressures of humans is best represented by a bell-shaped curve co...
Human Biology: Concepts and Current Issues (8th Edition)
What were the major microbiological interests of Martinus Beijerinck and Sergei Winogradsky? It can be said tha...
Brock Biology of Microorganisms (15th Edition)
1. Genetics affects many aspects of our lives. Identify three ways genetics affects your life or the life of a ...
Genetic Analysis: An Integrated Approach (3rd Edition)
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name the following molecules with IUpacarrow_forwardWhat is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forward
- How to get the predicted product of this reaction belowarrow_forwardPlease help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
Viscosity, Cohesive and Adhesive Forces, Surface Tension, and Capillary Action; Author: Professor Dave Explains;https://www.youtube.com/watch?v=P_jQ1B9UwpU;License: Standard YouTube License, CC-BY