
EBK ORGANIC CHEMISTRY
7th Edition
ISBN: 9780133556186
Author: Bruice
Publisher: VST
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11, Problem 89P
Triethylenemelamine (TEM) is an antitumor agent. Its activity is due to its ability to cross-link DNA.
- a. Explain why it can be used only under slightly acidic conditions.
- b. Explain why it can cross-link DNA.
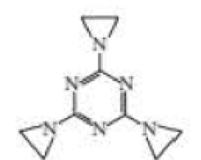
trithylenemelamine (TEM)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?
Indicate the formula of the product obtained by reacting D-Galactose with hydroxylamine.
Chemistry Question
Chapter 11 Solutions
EBK ORGANIC CHEMISTRY
Ch. 11.1 - Why are NH3 and CH3NH2 no longer nucleophiles when...Ch. 11.1 - Prob. 2PCh. 11.1 - Prob. 5PCh. 11.2 - Prob. 7PCh. 11.3 - Prob. 9PCh. 11.3 - Show how 1-propanol can be converted into the...Ch. 11.4 - Which of the following alcohols dehydrates the...Ch. 11.4 - Prob. 12PCh. 11.4 - Prob. 13PCh. 11.4 - Propose a mechanism for each of the following...
Ch. 11.4 - Draw the product of each of the following...Ch. 11.4 - Explain why the following alcohols, when heated...Ch. 11.4 - What stereoisomers are formed in the following...Ch. 11.4 - Prob. 18PCh. 11.4 - What alcohol would you treat with phosphorus...Ch. 11.5 - Prob. 20PCh. 11.6 - Prob. 22PCh. 11.7 - Prob. 24PCh. 11.7 - Would you expect the reactivity of a five-membered...Ch. 11.7 - Prob. 26PCh. 11.7 - What products are obtained from the reaction of...Ch. 11.7 - Prob. 28PCh. 11.7 - Prob. 29PCh. 11.7 - Prob. 30PCh. 11.8 - Prob. 31PCh. 11.8 - Prob. 32PCh. 11.8 - How do the major products obtained from...Ch. 11.8 - Explain why the two arene oxides in Problem 38...Ch. 11.8 - Three arene oxides can be obtained from...Ch. 11.9 - Explain why the half-life (the time it takes for...Ch. 11.10 - Prob. 38PCh. 11.10 - Prob. 39PCh. 11.10 - Prob. 40PCh. 11.10 - Prob. 41PCh. 11.10 - Prob. 42PCh. 11.11 - Using an alkyl halide and a thiol as starting...Ch. 11.11 - The following three nitrogen mustards were studied...Ch. 11.11 - Why is melphalan a good cancer drug?Ch. 11.11 - Prob. 47PCh. 11 - Prob. 48PCh. 11 - Which compound is more likely to be carcinogenic?Ch. 11 - Prob. 50PCh. 11 - Prob. 51PCh. 11 - Write the appropriate reagent over each arrow.Ch. 11 - What alkenes would you expect to be obtained from...Ch. 11 - Prob. 54PCh. 11 - When heated with H2SO4, both...Ch. 11 - What is the major product obtained from the...Ch. 11 - When deuterated phenanthrene oxide undergoes a...Ch. 11 - An unknown alcohol with a molecular formula of...Ch. 11 - Prob. 59PCh. 11 - Prob. 60PCh. 11 - Propose a mechanism for the following reaction:Ch. 11 - What product would be formed if the four-membered...Ch. 11 - Which of the following ethers would be obtained in...Ch. 11 - Using the given starting material any necessary...Ch. 11 - Prob. 65PCh. 11 - When 3-methyl-2-butanol is heated with...Ch. 11 - Propose a mechanism for each of the following...Ch. 11 - How could you synthesize isopropyl propyl ether,...Ch. 11 - When the following seven-membered ring alcohol is...Ch. 11 - Ethylene oxide reacts readily with HO because of...Ch. 11 - Prob. 71PCh. 11 - Propose a mechanism for each of the following...Ch. 11 - Explain why the acid-catalyzed dehydration of an...Ch. 11 - Triethylene glycol is one of the products obtained...Ch. 11 - Prob. 75PCh. 11 - Prob. 76PCh. 11 - When ethyl ether is heated with excess HI for...Ch. 11 - Propose a mechanism for the following reaction:Ch. 11 - Prob. 79PCh. 11 - An ion with a positively charged nitrogen atom in...Ch. 11 - Propose a mechanism for each of the following...Ch. 11 - Prob. 82PCh. 11 - The following reaction takes place several times...Ch. 11 - A vicinal diol has OH groups on adjacent carbons....Ch. 11 - Prob. 85PCh. 11 - Prob. 86PCh. 11 - Two stereoisomers are obtained from the reaction...Ch. 11 - Propose a mechanism for each or the following...Ch. 11 - Triethylenemelamine (TEM) is an antitumor agent....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
- 0/5 alekscgi/x/sl.exe/1o_u-IgNglkr7j8P3jH-IQs_pBaHhvlTCeeBZbufuBYTi0Hz7m7D3ZcSLEFovsXaorzoFtUs | AbtAURtkqzol 1HRAS286, O States of Matter Sketching a described thermodynamic change on a phase diagram The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 3 pressure (atm) + 0- 0 5+ 200 temperature (K) 400 Explanation Check X 0+ F3 F4 F5 F6 F7 S 2025 McGraw Hill LLC All Rights Reserved. Terms of Use Privacy Center Accessibility Q Search LUCR + F8 F9 F10 F11 F12 * % & ( 5 6 7 8 9 Y'S Dele Insert PrtSc + Backsarrow_forward5.arrow_forward9arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License