
Chemical Principles
8th Edition
ISBN: 9781305581982
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11, Problem 54E
Consider the galvanic cell based on the followinghalf-reactions:
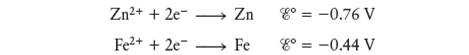
a. Determine the overall cell reaction and calculate 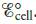
b. Calculate
c. Calculate 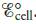 at 25°Cwhen
at 25°Cwhen
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
5. A buffer consists of 0.45 M NH, and 0.25 M NH-CI (PK of NH 474) Calculate the pH of the butter. Ans: 9.52
BAS
PH-9.26 +10g (10.95))
14-4.59
PH=4.52
6. To 500 ml of the buffer on #5 a 0.20 g of sample of NaOH was added
a Write the net ionic equation for the reaction which occurs
b. Should the pH of the solution increase or decrease sightly?
Calculate the pH of the buffer after the addition Ans: 9.54
Explain the inductive effect (+I and -I) in benzene derivatives.
The inductive effect (+I and -I) in benzene derivatives, does it guide ortho, meta or para?
Chapter 11 Solutions
Chemical Principles
Ch. 11 - Prob. 1DQCh. 11 - Prob. 2DQCh. 11 - You want to “plate out” nickel metal from a nickel...Ch. 11 - A copper penny can be dissolved in nitric acid but...Ch. 11 - Sketch a cell that forms iron metal from iron(II)...Ch. 11 - Which of the following is the best reducing agent:...Ch. 11 - You are told that metal A is a better reducing...Ch. 11 - Explain the following relationships: G and w, cell...Ch. 11 - Explain why cell potentials are not multiplied by...Ch. 11 - What is the difference between andWhen is equal to...
Ch. 11 - Prob. 11DQCh. 11 - Look up the reduction potential for Fe3+toFe2+ ....Ch. 11 - Prob. 13DQCh. 11 - Is the following statement true or false?...Ch. 11 - What is electrochemistry? What are redox...Ch. 11 - When magnesium metal is added to a beaker of...Ch. 11 - Prob. 17ECh. 11 - How can you construct a galvanic cell from two...Ch. 11 - Prob. 19ECh. 11 - Prob. 20ECh. 11 - Prob. 21ECh. 11 - Consider the following galvanic cells: For each...Ch. 11 - Prob. 23ECh. 11 - Prob. 24ECh. 11 - Answer the following questions using data from...Ch. 11 - Prob. 26ECh. 11 - Using data from Table 11.1, place the following in...Ch. 11 - Prob. 28ECh. 11 - Use the table of standard reduction potentials...Ch. 11 - Use the table of standard reduction potentials...Ch. 11 - Prob. 31ECh. 11 - A patent attorney has asked for your advice...Ch. 11 - The free energy change for a reaction G is an...Ch. 11 - The equation also can be applied to...Ch. 11 - Prob. 35ECh. 11 - Glucose is the major fuel for most living cells....Ch. 11 - Direct methanol fuel cells (DMFCs) have shown...Ch. 11 - The overall reaction and standard cell potential...Ch. 11 - Calculate the maximum amount of work that can...Ch. 11 - Prob. 40ECh. 11 - Prob. 41ECh. 11 - Chlorine dioxide (ClO2) , which is produced by...Ch. 11 - The amount of manganese in steel is determined...Ch. 11 - The overall reaction and equilibrium constant...Ch. 11 - Prob. 45ECh. 11 - Calculate for the reaction...Ch. 11 - A disproportionation reaction involves a substance...Ch. 11 - Calculate for the following half-reaction:...Ch. 11 - For the following half-reaction AlF63+3eAl+6F...Ch. 11 - Prob. 50ECh. 11 - The solubility product for CuI(s) is 1.11012....Ch. 11 - Explain the following statement: determines...Ch. 11 - Calculate the pH of the cathode compartment for...Ch. 11 - Consider the galvanic cell based on the...Ch. 11 - Prob. 55ECh. 11 - Consider the following galvanic cell at 25°C:...Ch. 11 - The black silver sulfide discoloration of...Ch. 11 - Consider the cell described below:...Ch. 11 - Consider the cell described below:...Ch. 11 - Prob. 60ECh. 11 - Prob. 61ECh. 11 - Prob. 62ECh. 11 - What are concentration cells? What is in a...Ch. 11 - A silver concentration cell is set up at 25°C as...Ch. 11 - Consider the concentration cell shown below....Ch. 11 - Prob. 66ECh. 11 - Prob. 67ECh. 11 - An electrochemical cell consists of a nickel metal...Ch. 11 - You have a concentration cell in which the cathode...Ch. 11 - Consider a galvanic cell at standard conditions...Ch. 11 - An electrochemical cell consists of a zinc metal...Ch. 11 - How long will it take to plate out each of the...Ch. 11 - What mass of each of the following substances can...Ch. 11 - It took 2.30 min with a current of 2.00 A to plate...Ch. 11 - The electrolysis of BiO+ produces pure bismuth....Ch. 11 - A single HallHeroult cell (as shown in Fig. 11.22)...Ch. 11 - A factory wants to produce 1.00103 kg barium...Ch. 11 - Why is the electrolysis of molten salts much...Ch. 11 - What reaction will take place at the cathode and...Ch. 11 - What reaction will take place at the cathode and...Ch. 11 - Prob. 81ECh. 11 - a. In the electrolysis of an aqueous solution of...Ch. 11 - A solution at 25°C contains 1.0 M...Ch. 11 - An aqueous solution of an unknown salt of...Ch. 11 - Consider the following half-reactions: A...Ch. 11 - An unknown metal M is electrolyzed. It took 74.1 s...Ch. 11 - Electrolysis of an alkaline earth metal chloride...Ch. 11 - Prob. 88ECh. 11 - What volume of F2 gas, at 25°C and 1.00 atm, is...Ch. 11 - Prob. 90ECh. 11 - In the electrolysis of a sodium chloride solution,...Ch. 11 - What volumes of H2(g)andO2(g) at STP are...Ch. 11 - Copper can be plated onto a spoon by placing the...Ch. 11 - Prob. 94AECh. 11 - Prob. 95AECh. 11 - Prob. 96AECh. 11 - Prob. 97AECh. 11 - Prob. 98AECh. 11 - Prob. 99AECh. 11 - Prob. 100AECh. 11 - Prob. 101AECh. 11 - Prob. 102AECh. 11 - Prob. 103AECh. 11 - Prob. 104AECh. 11 - In 1973 the wreckage of the Civil War ironclad...Ch. 11 - A standard galvanic cell is constructed so that...Ch. 11 - Prob. 107AECh. 11 - Prob. 108AECh. 11 - Prob. 109AECh. 11 - Prob. 110AECh. 11 - Prob. 111AECh. 11 - Prob. 112AECh. 11 - Prob. 113AECh. 11 - Consider a galvanic cell based on the following...Ch. 11 - Prob. 115AECh. 11 - Prob. 116AECh. 11 - Prob. 117AECh. 11 - Prob. 118AECh. 11 - Prob. 119CPCh. 11 - Prob. 120CPCh. 11 - A zinccopper battery is constructed as follows:...Ch. 11 - Prob. 122CPCh. 11 - Prob. 123CPCh. 11 - Prob. 124CPCh. 11 - Prob. 125CPCh. 11 - Prob. 126CPCh. 11 - Prob. 127CPCh. 11 - Prob. 128CPCh. 11 - Prob. 129CPCh. 11 - Prob. 130CPCh. 11 - Prob. 131CPCh. 11 - Prob. 132MPCh. 11 - Prob. 133MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 19.57 Using one of the reactions in this chapter, give the correct starting material (A-L) needed to produce each structure (a-f). Name the type of reaction used. (b) ہ مرد (d) HO (c) དང་ ་་ཡིན་ད་དང་ (f) HO Br B D of oli H J Br K C 人 ↑arrow_forwardInductive effect (+I and -I) in benzene derivatives.arrow_forward7. Helparrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Introduction to Electrochemistry; Author: Tyler DeWitt;https://www.youtube.com/watch?v=teTkvUtW4SA;License: Standard YouTube License, CC-BY