
OWLV2 FOR MOORE/STANITSKI'S CHEMISTRY:
5th Edition
ISBN: 9781285460369
Author: STANITSKI
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11, Problem 35QRT
The compound p-methoxybenzonitrile N-oxide, which has the formula CH3OC6H4CNO, reacts with itself to form a dimer—a molecule that consists of two p-methoxybenzonitrile N-oxide units connected to each other (CH3OC6H4CNO)2. The reaction can be represented as
A + A → B or 2 A → B
where A represents p-methoxybenzonitrile N-oxide and B represents the dimer, (CH3OC6H4CNO)2. For the reaction in carbon tetrachloride at 40 °C with an initial concentration of 0.011 M, these data were obtained:
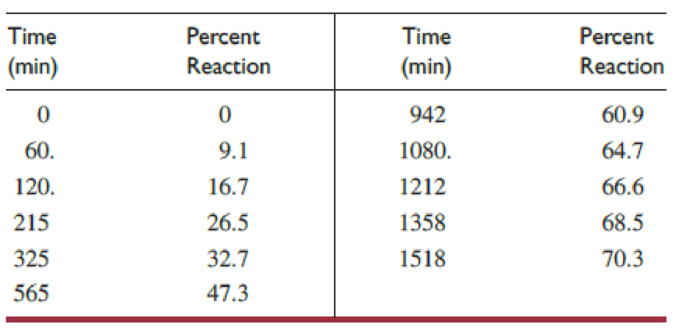
- (a) Determine the rate law for the reaction.
- (b) Determine the rate constant.
- (c) Determine the order of the reaction with respect to A.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
There are two molecules with the formula C3H§. Propene, CH;CH= CH2, is the monomer of the polymer
polypropylene, which is used for indoor-outdoor carpets. Cyclopropane is used as an anesthetic:
CH,
CH2
CH,
When heated to 499 °C, cyclopropane rearranges (isomerizes) and forms propene with a rate constant of
5.95 x 104s1. what is the half-life of this reaction? What fraction of the cyclopropane remains after 0.75 h at
499.5 °C?
6c12: In addition to solving the problem please give brief explanation for concepts and solution if able.
Phosgene (COCI) reacts with formaldehyde in the following reaction to produce dichloromethane and
carbon dioxide:
CoCI, + CH;0 → CH;Cl, + CO,
P+F>D+C
Activated charcoals have been found to catalyse this reaction at 170 °C (Ryan & Stacey, 1984).
One possible mechanism by which the reaction is thought to occur is shown below:
P+s = P.S
Egn 1
P.S + Fie
1.
C.S +D Egn 2
C.S
C+S
Egn 3
a) What type of reaction is Eqn 2 in the mechanism?
b) Write rate expressions (aka rate law aka isotherms) for all the reactions.
c) What do you believe would be the rate limiting step? Use this to derive the rate law for the reaction.
d) What is the best way to confirm the rate law you derived theoretically in c)?
Chapter 11 Solutions
OWLV2 FOR MOORE/STANITSKI'S CHEMISTRY:
Ch. 11.1 - For the reaction of crystal violet with NaOH(aq),...Ch. 11.1 - (a) From data in Table 11.1, calculate the rate of...Ch. 11.1 - For the reaction 4NO2(g)+O2(g)2N2O5(g) (a) express...Ch. 11.1 - Instantaneous rates for the reaction of hydroxide...Ch. 11.1 - Prob. 11.3CECh. 11.2 - Prob. 11.4ECh. 11.2 - Prob. 11.3PSPCh. 11.2 - Prob. 11.5ECh. 11.3 - Prob. 11.4PSPCh. 11.3 - Prob. 11.5PSP
Ch. 11.3 - Prob. 11.6PSPCh. 11.3 - Prob. 11.7PSPCh. 11.4 - Prob. 11.6ECh. 11.4 - Prob. 11.7CECh. 11.4 - Prob. 11.8PSPCh. 11.4 - Prob. 11.8CECh. 11.5 - Prob. 11.9PSPCh. 11.5 - The frequency factor A is 6.31 108 L mol1 s1 and...Ch. 11.6 - Prob. 11.10CECh. 11.7 - Prob. 11.11ECh. 11.7 - The Raschig reaction produces the industrially...Ch. 11.7 - Prob. 11.12ECh. 11.8 - The oxidation of thallium(I) ion by cerium(IV) ion...Ch. 11.9 - Prob. 11.11PSPCh. 11.9 - Prob. 11.14CECh. 11 - An excellent way to make highly pure nickel metal...Ch. 11 - Prob. 1QRTCh. 11 - Prob. 2QRTCh. 11 - Prob. 3QRTCh. 11 - Prob. 4QRTCh. 11 - Prob. 5QRTCh. 11 - Prob. 6QRTCh. 11 - Prob. 7QRTCh. 11 - Prob. 8QRTCh. 11 - Prob. 9QRTCh. 11 - Prob. 10QRTCh. 11 - Prob. 11QRTCh. 11 - Cyclobutane can decompose to form ethylene:
The...Ch. 11 - Prob. 13QRTCh. 11 - Prob. 14QRTCh. 11 - For the reaction 2NO2(g)2NO(g)+O2(g) make...Ch. 11 - Prob. 16QRTCh. 11 - Prob. 17QRTCh. 11 - Ammonia is produced by the reaction between...Ch. 11 - Prob. 19QRTCh. 11 - Prob. 20QRTCh. 11 - The reaction of CO(g) + NO2(g) is second-order in...Ch. 11 - Nitrosyl bromide, NOBr, is formed from NO and Br2....Ch. 11 - Prob. 23QRTCh. 11 - Prob. 24QRTCh. 11 - Prob. 25QRTCh. 11 - For the reaction
these data were obtained at 1100...Ch. 11 - Prob. 27QRTCh. 11 - Prob. 28QRTCh. 11 - Prob. 29QRTCh. 11 - Prob. 30QRTCh. 11 - Prob. 31QRTCh. 11 - Prob. 32QRTCh. 11 - For the reaction of phenyl acetate with water the...Ch. 11 - When phenacyl bromide and pyridine are both...Ch. 11 - The compound p-methoxybenzonitrile N-oxide, which...Ch. 11 - Prob. 36QRTCh. 11 - Radioactive gold-198 is used in the diagnosis of...Ch. 11 - Prob. 38QRTCh. 11 - Prob. 39QRTCh. 11 - Prob. 40QRTCh. 11 - Prob. 41QRTCh. 11 - Prob. 42QRTCh. 11 - Prob. 43QRTCh. 11 - Prob. 44QRTCh. 11 - Prob. 45QRTCh. 11 - Prob. 46QRTCh. 11 - Prob. 47QRTCh. 11 - Prob. 48QRTCh. 11 - Prob. 49QRTCh. 11 - Prob. 50QRTCh. 11 - Prob. 51QRTCh. 11 - Prob. 52QRTCh. 11 - For the reaction of iodine atoms with hydrogen...Ch. 11 - Prob. 54QRTCh. 11 - The activation energy Ea is 139.7 kJ mol1 for the...Ch. 11 - Prob. 56QRTCh. 11 - Prob. 57QRTCh. 11 - Prob. 58QRTCh. 11 - Prob. 59QRTCh. 11 - Prob. 60QRTCh. 11 - Prob. 61QRTCh. 11 - Prob. 62QRTCh. 11 - Prob. 63QRTCh. 11 - Which of the reactions in Question 62 would (a)...Ch. 11 - Prob. 65QRTCh. 11 - Prob. 66QRTCh. 11 - Prob. 67QRTCh. 11 - Prob. 68QRTCh. 11 - Prob. 69QRTCh. 11 - Prob. 70QRTCh. 11 - Prob. 71QRTCh. 11 - For the reaction the rate law is Rate=k[(CH3)3CBr]...Ch. 11 - Prob. 73QRTCh. 11 - Prob. 74QRTCh. 11 - Prob. 75QRTCh. 11 - For this reaction mechanism,
write the chemical...Ch. 11 - Prob. 77QRTCh. 11 - Prob. 78QRTCh. 11 - Prob. 79QRTCh. 11 - When enzymes are present at very low...Ch. 11 - Prob. 81QRTCh. 11 - The reaction is catalyzed by the enzyme succinate...Ch. 11 - Prob. 83QRTCh. 11 - Many biochemical reactions are catalyzed by acids....Ch. 11 - Prob. 85QRTCh. 11 - Prob. 86QRTCh. 11 - Prob. 87QRTCh. 11 - Prob. 88QRTCh. 11 - Prob. 89QRTCh. 11 - Prob. 90QRTCh. 11 - Prob. 91QRTCh. 11 - Prob. 92QRTCh. 11 - Prob. 93QRTCh. 11 - Prob. 94QRTCh. 11 - Nitryl fluoride is an explosive compound that can...Ch. 11 - Prob. 96QRTCh. 11 - Prob. 97QRTCh. 11 - For a reaction involving the decomposition of a...Ch. 11 - Prob. 99QRTCh. 11 - Prob. 100QRTCh. 11 - Prob. 101QRTCh. 11 - This graph shows the change in concentration as a...Ch. 11 - Prob. 103QRTCh. 11 - Prob. 104QRTCh. 11 - Prob. 105QRTCh. 11 - Prob. 106QRTCh. 11 - Prob. 107QRTCh. 11 - Prob. 108QRTCh. 11 - Prob. 109QRTCh. 11 - Prob. 110QRTCh. 11 - Prob. 111QRTCh. 11 - Prob. 112QRTCh. 11 - Prob. 113QRTCh. 11 - Prob. 114QRTCh. 11 - Prob. 115QRTCh. 11 - Prob. 116QRTCh. 11 - Prob. 118QRTCh. 11 - Prob. 119QRTCh. 11 - In a time-resolved picosecond spectroscopy...Ch. 11 - If you know some calculus, derive the integrated...Ch. 11 - If you know some calculus, derive the integrated...Ch. 11 - (Section 11-5) A rule of thumb is that for a...Ch. 11 - Prob. 11.BCPCh. 11 - Prob. 11.CCPCh. 11 - Prob. 11.DCP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Silicon forms a series of compounds analogous to the al-kanes and having the general formula SinH2n+2. The first of these compounds is silane, SiH4, which is used in the electronics industry to produce thin ultrapure silicon films. SiH4(g) is somewhat difficult to work with because it is py-ropboric at room temperature—meaning that it bursts into flame spontaneously when exposed to air. (a) Write an equation for the combustion of SiH4(g). (The reaction is analogous to hydrocarbon combustion, and SiO2 is a solid under standard conditions. Assume the water produced will be a gas.) (b) Use the data from Appendix E to calculate ? for this reaction. (c) Calculate G and show that the reaction is spontaneous at 25°C. (d) Compare G for this reaction to the combustion of methane. (See the previous problem.) Are the reactions in these two exercises enthalpy or entropy driven? Explain.arrow_forwardOne mechanism for the destruction of ozone in the upper atmosphere is a. Which species is a catalyst? b. Which species is an intermediate? c. Ea for the uncatalyzed reaction O3(g)+O(g)2O2(g) is 14.0 kJ. Ea. for the same reaction when catalyzed is 11.9 kJ. What is the ratio of the rate constant for the catalyzed reaction to that for the uncatalyzed reaction at 25C? Assume that the frequency factor A is the same for each reaction.arrow_forwardG.49.arrow_forward
- Which of the following reactions would you predict to have the largest orientation factor? A) NOF (g) + NOF (g) → 2NO (g) + F2 (g) B) NH3 (g) + BCl3 (g) → H3N-BCl3 (g) C) Br2 (g) + H2C=CH2 (g) → H2BrC-CBrH2 (g) D) H (g) + Cl (g) → HCl (g) E) All of these reactions should have nearly identical orientation factors.arrow_forwardwrite a rate law for the following reaction if reaction order n=1 2 N2O5 (g) -> 4 NO2 (g) + O2 (g)arrow_forwardThe enzyme carbonic anhydrase catalyzes the following reaction: CO2 (g) + H2O(1) - HCO3 (aq) +H+(aq) -1 In water, without the enzyme, the reaction proceeds with a rate constant of 0.039 s at 24 °C. In the presence of the enzyme in water, the reaction proceeds with a rate constant of 1.0 ×106 s-1 at 24 °C. Part A Assuming the collision factor is the same for both situations, calculate the difference in activation energies for the uncatalyzed (Ea) versus the enzyme-catalyzed (Eac) reaction. Express your answer in kilojoules to two significant figures. Ea – Eac = kJ Submit Request Answerarrow_forward
- The rate constant of a chemical reaction increased from 0.100 s−1s−1 to 2.60 s−1s−1 upon raising the temperature from 25.0 ∘C∘C to 53.0 ∘C∘C . A. Calculate the value of (1T2−1T1)(1T2−1T1) where T1T1T_1 is the initial temperature and T2T2T_2 is the final temperature. Express your answer numerically. B. Calculate the value of ln(k1k2)ln(k1k2) where k1k1k_1 and k2k2k_2 correspond to the rate constants at the initial and the final temperatures as defined in part A. C. What is the activation energy of the reaction? Express your answer numerically in kilojoules per mole.arrow_forwardThe formation of dinitrogen pentoxide is described by the following chemical equation: 2NO, (g) + O; (g) → 0, (g) + N,O5 (g) Suppose a two-step mechanism is proposed for this reaction, beginning with this elementary reaction: NO2 (g) + 03 (g) NO; (g) + 0, Suppose also that the second step of the mechanism should be bimolecular. Suggest a reasonable second step. That is, write the balanced chemical equation of a bimolecular elementary reaction that would complete the proposed mechanism.arrow_forwardSuppose the formation of tert-butanol proceeds by the following mechanism: step elementary reaction rate constant 1 CH33CBr (aq) → CH33C+ (aq) + Br− (aq) k1 2 CH33C+ (aq) + OH− (aq) → CH33COH (aq) k2 Suppose also k1 ≫ k2 . That is, the first step is much faster than the second.arrow_forward
- Ethylene oxide is a reagent important in many chemical processes and also a major component in explosives. It is formed through the partial oxidation of ethylene: C2H4 + 1/2 O2 → C2H4O k₁ = 5665 s-1 However, ethylene can also undergo a combustion reaction: C2H4 + 3/2 O2 → CO₂ + H₂O k2 = ? s-1 Assuming both reactions obey first order kinetics with excess oxygen and that after 9.6 minutes [C2H4O]/[CO2] = 7.9, what is the rate constant k2? In water, the fluorescence quantum yield and observed excited-state lifetime of tryptophan are Of = 0.19 and to = 2.6 ns, respectively. What is the fluorescence rate constant (in s-1) of tryptophan?arrow_forward1 Rate constants for the first-order decomposition of acetonedicarboxylic acid CO(CH2COOH)2(aq) → CO(CH3)2(aq) + 2 CO2(g) acetonedicarboxylic acidacetone are k = 4.75 ×10–4 s–1 at 293 K and k = 1.63 ×10–3 at 303 K. What is the activation energy, Ea, for this reaction? Select one: a. 71KJ/mol b. 81KJ/mol c. 51KJ/mol d. 91kJ/molarrow_forwardA study of the rate of dimerization of C4H6 gave the data shown in the table: 2C4H6→C8H12 Time (s) [C4H6] (M) 0 1.00 x 10–2 1600 5.04 x 10–3 3200 3.37 x 10–3 4800 2.53 x 10–3 6200 2.08 x 10–3 What is the instantaneous rate of dimerization at 3200 s? Create a graph of time versus [C4H6] to help answer this question. Question 1 options: a) 9.4 x 10-7 M s-1 b) 8.2 x 10-7 M s-1 c) 7.7 x 10-7 M s-1 d) 6.5 x 10-7 M s-1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
Kinetics: Initial Rates and Integrated Rate Laws; Author: Professor Dave Explains;https://www.youtube.com/watch?v=wYqQCojggyM;License: Standard YouTube License, CC-BY