
Concept explainers
Give an acceptable IUPAC name for each of the following compounds:
Interpretation:
The acceptable IUPAC name for each of the given compounds is to be deteremined.
Concept introduction:
Alkanes have the general formula
The parent chain in each of hydrocarbon structure is first identified and the chain is numbered such that the substituents attached get lowest numbers.
Prefixes are used to denote the number of identical substituents.
The substituents are written in alphabetical order while writing the IUPAC name.
If the two groups of atoms that are the higher priority are on the same side of the double bond, then the bond is termed as the Z-configuration. While if the two group of higher priority are on the opposite side then the configuration is E-configuration.
Answer to Problem 27P
Solution:
a)
b)
c)
d)
e)
f)
g)
h)
Explanation of Solution
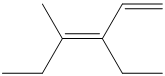
a) The expanded structure for the given structure can be drawn as follows:
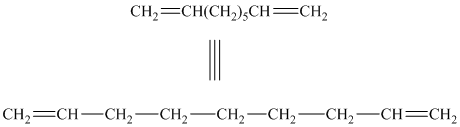
In the above structure, the parent chain is the continuous chain from
Thus, the IUPAC name is
b) The structure for the given compound is as follows:
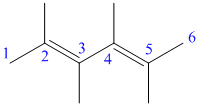
In the above structure, the parent longest chain is from
Thus, the IUPAC name is
c) The structure for the given compound and its expanded structure is as follows:
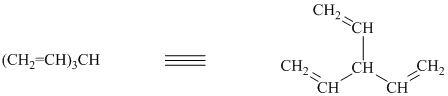
In the above structure, the parent longest chain is from
Thus, the IUPAC name is
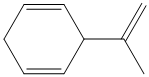
d) The structure for the given compound is as follows:
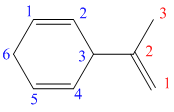
In the above structure, the parent ring contains six carbon atoms, so the parent name of the cycloalkane is cyclohexane. There are two double bonds in the given compound, the first one exist in between
Considering the Cahn–Ingold–Prelog sequence rules, for the cyclohexadiene, the higher priority groups are on the same side in both the double bonds. Hence, both get the stereochemical label as Z.
Thus, the IUPAC name of this structure is
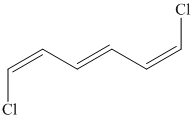
e) The structure for the given compound is as follows:

In the above structure, the parent longest chain is from
For double bonds at
For the internal double bond at carbon
Thus, the IUPAC name is
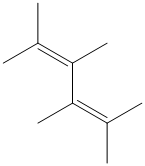
f) The structure for the given compound is as follows:
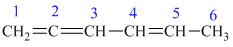
In the above structure, the parent longest chain is from
Thus, the IUPAC name is
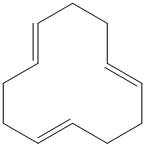
g) The structure for the given compound is as follows:
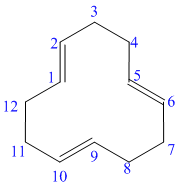
In the above structure, the parent ring contains twelve carbon atoms, so the parent name of the cycloalkane is cyclododecane. There are three double bonds in the given compound, the first one exist in between
For all the three double bonds at
Thus, the IUPAC name of this structure is
h) The structure for the given compound is as follows:
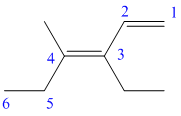
In the above structure, the parent longest chain is from
For the double bonds at
Thus, the IUPAC name is
Want to see more full solutions like this?
Chapter 11 Solutions
Organic Chemistry - Standalone book
- The emission data in cps displayed in Table 1 is reported to two decimal places by the chemist. However, the instrument output is shown in Table 2. Table 2. Iron emission from ICP-AES Sample Blank Standard Emission, cps 579.503252562 9308340.13122 Unknown Sample 343.232365741 Did the chemist make the correct choice in how they choose to display the data up in Table 1? Choose the best explanation from the choices below. No. Since the instrument calculates 12 digits for all values, they should all be kept and not truncated. Doing so would eliminate significant information. No. Since the instrument calculates 5 decimal places for the standard, all of the values should be limited to the same number. The other decimal places are not significant for the blank and unknown sample. Yes. The way Saman made the standards was limited by the 250-mL volumetric flask. This glassware can report values to 2 decimal places, and this establishes our number of significant figures. Yes. Instrumental data…arrow_forwardSteps and explanation pleasearrow_forwardSteps and explanation to undertand concepts.arrow_forward
- Nonearrow_forward7. Draw a curved arrow mechanism for the following reaction. HO cat. HCI OH in dioxane with 4A molecular sievesarrow_forwardTry: Convert the given 3D perspective structure to Newman projection about C2 - C3 bond (C2 carbon in the front). Also, show Newman projection of other possible staggered conformers and circle the most stable conformation. Use the template shown. F H3C Br Harrow_forward
- Nonearrow_forward16. Consider the probability distribution p(x) = ax", 0 ≤ x ≤ 1 for a positive integer n. A. Derive an expression for the constant a, to normalize p(x). B. Compute the average (x) as a function of n. C. Compute σ2 = (x²) - (x)², the variance of x, as a function of n.arrow_forward451. Use the diffusion model from lecture that showed the likelihood of mixing occurring in a lattice model with eight lattice sites: Case Left Right A B C Permeable Barrier → and show that with 2V lattice sites on each side of the permeable barrier and a total of 2V white particles and 2V black particles, that perfect de-mixing (all one color on each side of the barrier) becomes increasingly unlikely as V increases.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
