
When boron trifluoride reacts with ammonia, the following reaction occurs:
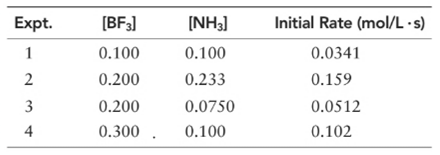
(a) What is the order of the reaction with respect to BF3, NH3, and overall?
(b) Write the rate expression for the reaction.
(c) Calculate k for the reaction.
(d) When
(a)
Interpretation:
To determine the order of reaction with respect to BF3, NH3 and overall for the following reaction:
Concept introduction:
Rate of a chemical reaction: It tells us about the speed at which the reactants are converted into products.
Mathematically, rate of reaction is directly proportional to the product of concentration of each reactant raised to the power equal to their respective stoichiometric coefficients.
Let’s say we have a reaction:
Answer to Problem 25QAP
Order of given reaction
With respect to BF3 =1
With respect to NF3 =1
Overall = 2.
Explanation of Solution
Given information:
Here the chemical reaction is:

Let’s assume the reaction to be ‘t’ order with respect to BF3 and ‘y’ order with respect to NH3.
Then, rate law for experiment 1 in above reaction will be:
And, rate law for experiment 4 in above reaction will be;
Divide (1) by (2) to get value of ‘t’.
Now writing rate law for experiment 2 in above reaction will be;
And, rate law for experiment 4 in above reaction will be;
Divide (3) by (4) to get value of ‘y’.
Thus, order with respect to NH3 is 1.
And the order of reaction will be:
Thus, overall order of reaction is 2.
(b)
Interpretation:
To write the rate expression for the given reaction.
Concept introduction:
Rate of a chemical reaction: It tells us about the speed at which the reactants are converted into products.
Mathematically, rate of reaction is directly proportional to the product of concentration of each reactant raised to the power equal to their respective stoichiometric coefficients.
Let’s say we have a reaction:
Answer to Problem 25QAP
Rate law expression for the given reaction will be;
Explanation of Solution
Here the chemical reaction is:
Order of reaction with respect to BF3 = 1
Order of reaction with respect to NH3 = 1
Let the rate constant be ‘k’.
Then, rate law expression for above reaction will be;
(c)
Interpretation:
To determine the rate constant for the given reaction.
Concept introduction:
Rate of a chemical reaction: It tells us about the speed at which the reactants are converted into products.
Mathematically, rate of reaction is directly proportional to the product of concentration of each reactant raised to the power equal to their respective stoichiometric coefficients.
Let’s say we have a reaction:
Answer to Problem 25QAP
Rate constant for given reaction is
Explanation of Solution
Here the chemical reaction is:
Rate law expression for above reaction:
Plugging values in rate law expression for experiment 1 as:
Hence, the rate constant is
(d)
Interpretation:
To determine the rate of reaction at given concentration of reactants.
Concept introduction:
Rate of a chemical reaction: It tells us about the speed at which the reactants are converted into products.
Mathematically, rate of reaction is directly proportional to the product of concentration of each reactant raised to the power equal to their respective stoichiometric coefficients.
Let’s say we have a reaction:
Answer to Problem 25QAP
Rate of reaction for given reaction at given conditions is
Explanation of Solution
Here the chemical reaction is:
Rate law expression for above reaction:
Here we have:
[BF3 ]= 0.533 M
[NH3 ] = 0.300 M
Rate constant = 3.41 L/mol.s
Plugging values in rate law as:
Hence, the rate of reaction is
Want to see more full solutions like this?
Chapter 11 Solutions
Bundle: Chemistry: Principles and Reactions, 8th, Loose-Leaf + OWLv2, 1 term (6 months) Printed Access Card
- Explanation O Conjugated Pi Systems Deducing the reactants of a Diels-Alder reaction Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. Click and drag to start drawing a structure. Xarrow_forwardDiels Alder Cycloaddition: Focus on regiochemistry (problems E-F) –> match + of thedienophile and - of the diene while also considering stereochemistry (endo).arrow_forwardHELP! URGENT! PLEASE RESOND ASAP!arrow_forward
- Question 4 Determine the rate order and rate constant for sucrose hydrolysis. Time (hours) [C6H12O6] 0 0.501 0.500 0.451 1.00 0.404 1.50 0.363 3.00 0.267 First-order, k = 0.210 hour 1 First-order, k = 0.0912 hour 1 O Second-order, k = 0.590 M1 hour 1 O Zero-order, k = 0.0770 M/hour O Zero-order, k = 0.4896 M/hour O Second-order, k = 1.93 M-1-hour 1 10 ptsarrow_forwardDetermine the rate order and rate constant for sucrose hydrolysis. Time (hours) [C6H12O6] 0 0.501 0.500 0.451 1.00 0.404 1.50 0.363 3.00 0.267arrow_forwardDraw the products of the reaction shown below. Use wedge and dash bonds to indicate stereochemistry. Ignore inorganic byproducts. OSO4 (cat) (CH3)3COOH Select to Draw ઘarrow_forward
- Calculate the reaction rate for selenious acid, H2SeO3, if 0.1150 M I-1 decreases to 0.0770 M in 12.0 minutes. H2SeO3(aq) + 6I-1(aq) + 4H+1(aq) ⟶ Se(s) + 2I3-1(aq) + 3H2O(l)arrow_forwardProblem 5-31 Which of the following objects are chiral? (a) A basketball (d) A golf club (b) A fork (c) A wine glass (e) A spiral staircase (f) A snowflake Problem 5-32 Which of the following compounds are chiral? Draw them, and label the chirality centers. (a) 2,4-Dimethylheptane (b) 5-Ethyl-3,3-dimethylheptane (c) cis-1,4-Dichlorocyclohexane Problem 5-33 Draw chiral molecules that meet the following descriptions: (a) A chloroalkane, C5H11Cl (c) An alkene, C6H12 (b) An alcohol, C6H140 (d) An alkane, C8H18 Problem 5-36 Erythronolide B is the biological precursor of erythromycin, a broad-spectrum antibiotic. How H3C CH3 many chirality centers does erythronolide B have? OH Identify them. H3C -CH3 OH Erythronolide B H3C. H3C. OH OH CH3arrow_forwardPLEASE HELP! URGENT! PLEASE RESPOND!arrow_forward
- 2. Propose a mechanism for this reaction. ہلی سے ملی N H (excess)arrow_forwardSteps and explanationn please.arrow_forwardProblem 5-48 Assign R or S configurations to the chirality centers in ascorbic acid (vitamin C). OH H OH HO CH2OH Ascorbic acid O H Problem 5-49 Assign R or S stereochemistry to the chirality centers in the following Newman projections: H Cl H CH3 H3C. OH H3C (a) H H H3C (b) CH3 H Problem 5-52 Draw the meso form of each of the following molecules, and indicate the plane of symmetry in each: OH OH (a) CH3CHCH2CH2CHCH3 CH3 H3C. -OH (c) H3C CH3 (b) Problem 5-66 Assign R or S configurations to the chiral centers in cephalexin, trade-named Keflex, the most widely prescribed antibiotic in the United States. H2N H IHH S Cephalexin N. CH3 CO₂Harrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





