
Bundle: Chemistry: Principles and Reactions, 8th, Loose-Leaf + OWLv2, 1 term (6 months) Printed Access Card
8th Edition
ISBN: 9781305717497
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11, Problem 23QAP
For a reaction involving the decomposition of Z at a certain temperature, the following data are obtained:
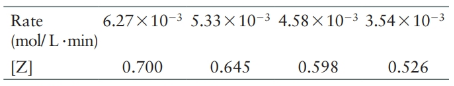
(a) What is the order of the reaction?
(b) Write the rate expression for the decomposition of Z.
(c) Calculate k for the decomposition at that temperature.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which of the following orbitals intersect or overlap the x-axis in the standard cartesian coordinate system used? (Select ALL correct answers.)
Group of answer choices
px
dxz
dx2-y2
py
dxy
s
Which of the following sets of elements is not a Dobereiner triad? (Choose the best answer.) Group of answer choices
Li-Na-K
Al-Ga-In
Cr-Mo-W
K-Rb-Cs
Don't used Ai solution and don't used hand raiting
Chapter 11 Solutions
Bundle: Chemistry: Principles and Reactions, 8th, Loose-Leaf + OWLv2, 1 term (6 months) Printed Access Card
Ch. 11 - Express the rate of the reaction...Ch. 11 - Express the rate of the reaction...Ch. 11 - Consider the following hypothetical reaction: X( g...Ch. 11 - Consider the following hypothetical reaction:...Ch. 11 - Consider the combustion of ethane:...Ch. 11 - For the reaction 5Br(aq)+BrO3(aq)+6...Ch. 11 - Nitrosyl chloride (NOCI) decomposes to nitrogen...Ch. 11 - Ammonia is produced by the reaction between...Ch. 11 - Experimental data are listed for the following...Ch. 11 - Experimental data are listed for the hypothetical...
Ch. 11 - A reaction has two reactants X and Y. What is the...Ch. 11 - A reaction has two reactants Q and P. What is the...Ch. 11 - What will the units of the rate constants in...Ch. 11 - What will the units of the rate constants in...Ch. 11 - Consider the reaction ZproductsThe data below give...Ch. 11 - Consider the reaction YproductsThe graph below...Ch. 11 - Complete the following table for the reaction...Ch. 11 - Complete the following table for the reaction...Ch. 11 - The decomposition of nitrogen dioxide is a...Ch. 11 - The decomposition of ammonia on tungsten at 1100C...Ch. 11 - The reaction ICl(g)+12 H2(g)12 I2(g)+HCl(g)is...Ch. 11 - The hypothetical reaction X(g)+12Y(g)productsis...Ch. 11 - For a reaction involving the decomposition of Z at...Ch. 11 - For a reaction involving the decomposition of Y,...Ch. 11 - When boron trifluoride reacts with ammonia, the...Ch. 11 - When nitrogen dioxide reacts with carbon monoxide,...Ch. 11 - Hydrogen bromide is a highly reactive and...Ch. 11 - Diethylhydrazine reacts with iodine according to...Ch. 11 - The equation for the reaction between iodide and...Ch. 11 - Prob. 30QAPCh. 11 - In a solution at a constant H+ concentration,...Ch. 11 - Consider the reaction Â...Ch. 11 - Nitrosyl bromide decomposes to nitrogen oxide and...Ch. 11 - Prob. 34QAPCh. 11 - Azomethane decomposes into nitrogen and ethane at...Ch. 11 - The decomposition of sulfuryl chloride, SO2Cl2, to...Ch. 11 - The first-order rate constant for the...Ch. 11 - Consider the first-order decomposition of phosgene...Ch. 11 - The decomposition of azomethane, (CH3)2N2, to...Ch. 11 - The first-order rate constant for the...Ch. 11 - In the first-order decomposition of acetone at...Ch. 11 - The decomposition of sulfuryl chlorideSO2Cl2fur...Ch. 11 - Dinitrogen pentoxide gas decomposes to form...Ch. 11 - Sucrose (C12H22O11) hydrolyzes into glucose and...Ch. 11 - Iodine-131 is used to treat tumors in the thyroid....Ch. 11 - Cesium-131 is the latest tool of nuclear medicine....Ch. 11 - Prob. 47QAPCh. 11 - A sample of sodium-24 chloride contains 0.050 mg...Ch. 11 - The decomposition of A at 850C is a zero-order...Ch. 11 - The decomposition of R at 33C is a zero-order...Ch. 11 - For the zero-order decomposition of HI on a gold...Ch. 11 - For the zero-order decomposition of ammonia on...Ch. 11 - Ammonium cyanate, NH4NCO, in water rearranges to...Ch. 11 - Butadiene, C4H6, dimerizes according to the...Ch. 11 - The rate constant for the second-order reaction...Ch. 11 - The decomposition of nitrosyl chloride...Ch. 11 - An increase in temperature from 23C to 36C...Ch. 11 - If the activation energy of a reaction is 9.13 kJ,...Ch. 11 - The following data are obtained for the gas-phase...Ch. 11 - The following data are obtained for the...Ch. 11 - Consider the following hypothetical reaction:...Ch. 11 - For the reaction: Q+RY+ZH=128kJ Draw a...Ch. 11 - The uncoiling of deoxyribonucleic acid (DNA) is a...Ch. 11 - The precipitation of egg albumin in water at 100C...Ch. 11 - Prob. 65QAPCh. 11 - Prob. 66QAPCh. 11 - For the reaction 2N2O(g)2N2(g)+O2(g) the rate...Ch. 11 - For the decomposition of a peroxide, the...Ch. 11 - Consider a 5.000 M solution of the hypothetical...Ch. 11 - The decomposition of N2O5 to NO2 and NO3 is a...Ch. 11 - For a certain reaction, Ea is 135 kJ and H=45 kJ....Ch. 11 - Consider a reaction in which E a=129 kJ and H=29...Ch. 11 - A catalyst lowers the activation energy of a...Ch. 11 - A reaction has an activation energy of 363 kJ at...Ch. 11 - Write the rate expression for each of the...Ch. 11 - Write the rate expression for each of the...Ch. 11 - For the reaction between hydrogen and iodine,...Ch. 11 - For the reaction 2H2(g)+2NO(g)N2(g)+2H2O(g) the...Ch. 11 - At low temperatures, the rate law for the reaction...Ch. 11 - Two mechanisms are proposed for the reaction...Ch. 11 - The hypothetical reaction QR+Xproductswas...Ch. 11 - When a base is added to an aqueous solution of...Ch. 11 - The decomposition of sulfuryl chloride, SO2Cl2, to...Ch. 11 - How much faster would a reaction proceed at 46C...Ch. 11 - Prob. 85QAPCh. 11 - Prob. 86QAPCh. 11 - A drug decomposes in the blood by a first-order...Ch. 11 - Prob. 88QAPCh. 11 - Prob. 89QAPCh. 11 - Prob. 90QAPCh. 11 - Consider the decomposition of A represented by...Ch. 11 - Consider the decomposition reaction 2X2Y+ZThe...Ch. 11 - Consider the following activation energy diagram....Ch. 11 - Three first-order reactions have the following...Ch. 11 - Consider the first-order decomposition reaction...Ch. 11 - Consider the following energy diagram (not to...Ch. 11 - Prob. 97QAPCh. 11 - Prob. 98QAPCh. 11 - The gas-phase reaction between hydrogen and iodine...Ch. 11 - Consider the coagulation of a protein at 100C. The...Ch. 11 - Prob. 101QAPCh. 11 - Prob. 102QAPCh. 11 - Prob. 103QAPCh. 11 - In a first-order reaction, suppose that a quantity...Ch. 11 - Consider the hypothetical first-order reaction...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Don't used hand raiting and don't used Ai solutionarrow_forwardGive the structure(s) of the product(s) the reaction below, and be sure to indicate any relative stereochemistry (you can assume that each of the Diels-Alder reactions will proceed with endo selectivity). Draw out relevant enantiomer(s) if they are expected to form. If no reaction is expected to occur under the indicated conditions, then write "no reaction" or NR, and explain why you would expect nothing to occur. If more than one product is formed, please indicate which one will be the major product or if they will be formed in equal amounts. In all cases, equimolar amounts of both components/reagents are present unless indicated otherwise I'm struggling to see how this reaction will go! I am wondering if it will cycle on itself but I'm not sure how I drew out a decagon but I'm a bit lostarrow_forwardGive the structure(s) of the product(s) for the reactions below, and be sure to indicate any relative stereochemistry (you can assume that each of the Diels-Alder reactions will proceed with endo selectivity). Draw out relevant enantiomer(s) if they are expected to form. If no reaction is expected to occur under the indicated conditions, then write "no reaction" or NR, and explain why you would expect nothing to occur. If more than one product is formed, please indicate which one will be the major product or if they will be formed in equal amounts. In all cases, equimolar amounts of both components/reagents are present unless indicated otherwise .arrow_forward
- Calculate the residence time of strontium (Sr2+) in the world ocean, given that the average concentration of strontium in the world’s rivers is approximately 0.87 µmol L-1 (5 pts).arrow_forwardA package contains 1.33lbs of ground round. If it contains 29% fat, how many grams of fat are in the ground? arrow_forwardHow is the resonance structure formed to make the following reaction product. Please hand draw the arrows showing how the electrons move to the correct position. Do not use an AI answer. Please draw it yourself or don't bother.arrow_forward
- Part II Calculate λ max of the following compounds using wood ward- Fiecer rules a) b) c) d) e) OH OH dissolved in dioxane Br Br dissolved in methanol. NH₂ OCH 3 OHarrow_forward6. Match each of the lettered items in the column on the left with the most appropriate numbered item(s) in the column on the right. Some of the numbered items may be used more than once and some not at all. a. Z = 37 1. b. Mn 2. C. Pr element in period 5 and group 14 element in period 5 and group 15 d. S e. [Rn] 7s¹ f. d block metal 3. highest metallic character of all the elements 4. paramagnetic with 5 unpaired electrons 5. 4f36s2 6. isoelectronic with Ca²+ cation 7. an alkaline metal 8. an f-block elementarrow_forwardDraw all formal charges on the structures below as is and draw 1 resonance structure that is more stable.arrow_forward
- Part II. xiao isolated a compound TAD (Ca H 10 N₂) from tobacco and obtained its IR spectrum. Xiao proposed a chemical structure shown below: % Transmittance 4000 3500 3000 2500 2000 Wavenumber (cm-1) 1500 1000 (a) Explain why her proposed structure is inconsistent with the IR spectrum obtained (b) TAD exists as a tautomer of the structure xiao proposed. Draw the structure and explain why it is more compatible with the obtained spectrum. (C) what is the possible source for the fairly intense signal at 1621cm1arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32; Author: Crash Course;https://www.youtube.com/watch?v=7qOFtL3VEBc;License: Standard YouTube License, CC-BY