
Equilibrium vapor pressures of benzene, C6H6, at various temperatures are given in the table.
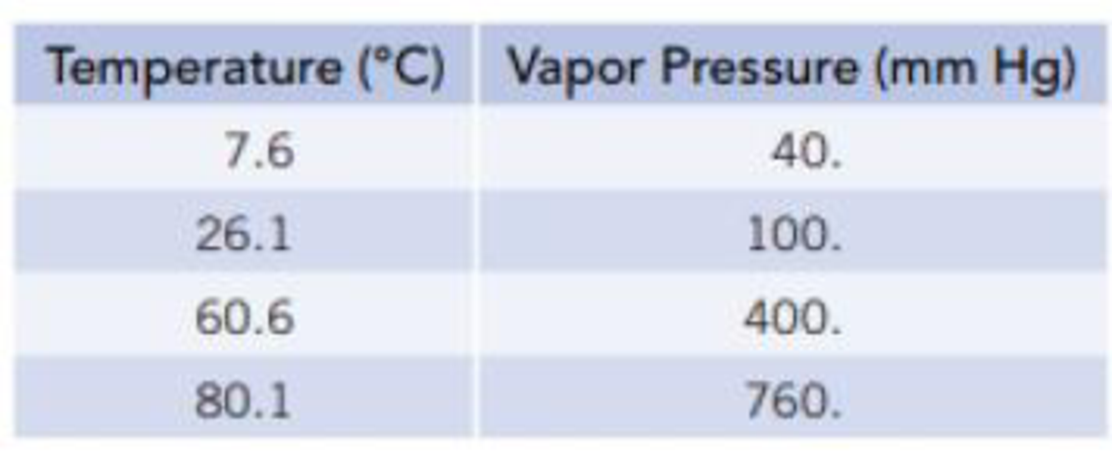
- (a) What is the normal boiling point of benzene?
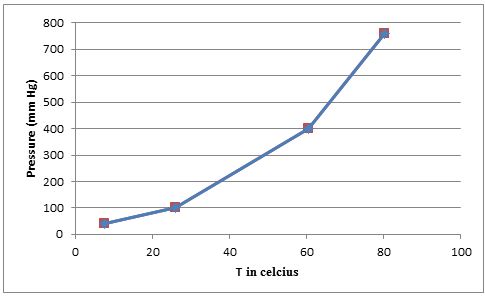
- (b) Plot these data so that you have a graph resembling the one in Figure 11.12. At what temperature does the liquid have an equilibrium vapor pressure of 250 mm Hg? At what temperature is the vapor pressure 650 mm Hg?
- (c) Calculate the molar enthalpy of vaporization for benzene using the Clausius–Clapeyron equation.
(a)
Interpretation:
The normal boiling point of benzene has to be determined.
Concept Introduction:
Boiling point: It is the temperature at which liquid converts to vapor. At boiling point the vapor pressure of liquid and the pressure of the surroundings are equal.
Normal boiling point: When the external pressure is
Answer to Problem 21PS
The normal boiling point of benzene is
Explanation of Solution
The normal boiling point of benzene is calculated
Given:
Normal boiling point is the temperature when the external pressure is
From the given data it is clear that the temperature at which the pressure is
Thus the normal boiling point of benzene is
(b)
Interpretation:
The temperature versus vapor pressure graph should be plotted. The temperatures at which the liquid has vapour pressures of
Concept Introduction:
Vapor pressure is nothing but the pressure of a vapor in contact with its liquid or solid form.
When a liquid and vapor are in equilibrium the pressure exerted by the vapor is called the equilibrium vapor pressure
Answer to Problem 21PS
The temperatures at which liquid have a vapour pressures of
Explanation of Solution
Given,
The temperatures at which liquid have a vapour pressures of
Using the given data we can plot the graph of
From the graph we can find the approximate temperatures at which the pressures are
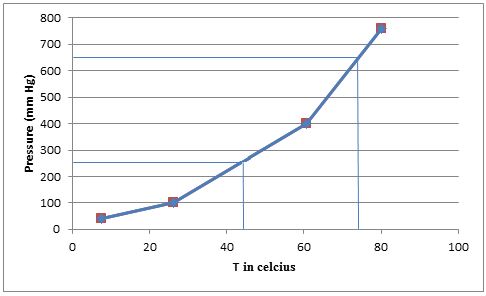
Therefore,
The temperature at which the pressure is
(c)
Interpretation:
The molar enthalpy of vaporization using Clausius-Clapeyron has to be determined
Concept Introduction:
Clausius-Clapeyron equation:
From this relationship we can calculate the molar enthalpy of vaporization by knowing the corresponding temperature and pressure values.
If we have pressures at two different temperatures, then enthalpy of vaporization can be calculated by
Answer to Problem 21PS
The molar enthalpy of vaporization of is
Explanation of Solution
The molar enthalpy of vaporization is calculated using the given data,
Given:
Clausius-Clapeyron equation is,
Substituting the values
The molar enthalpy of vaporization of is
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry & Chemical Reactivity
Additional Science Textbook Solutions
Organic Chemistry (8th Edition)
Campbell Essential Biology with Physiology (5th Edition)
Organic Chemistry
Loose Leaf For Integrated Principles Of Zoology
Chemistry: Structure and Properties (2nd Edition)
- Hi can you please help me solve this problem? thank youarrow_forwardAn electrode process takes place at a metal-solution interface. Indicate the current condition that must be met for Faradaic rectification to occur.arrow_forwardAt a metal-solution interface, an electron is exchanged, and the symmetry factor beta < 0.5 is found in the Butler-Volmer equation. What does this indicate?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





