
(a)
Interpretation: The galvanic cells based on given overall reaction needs to be sketched and the value of
Concept Introduction: A galvanic cell is an electro chemical cell that drives electrical energy from
In a galvanic cell the oxidation and the reduction half cells are separated by a connecting wire so that electrons can flow through the wire.
(a)
Answer to Problem 19E
Sketch of a galvanic cell is as follows:
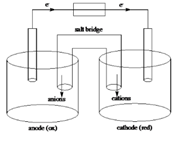
Values of
Explanation of Solution
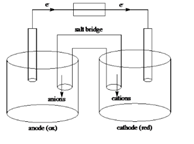
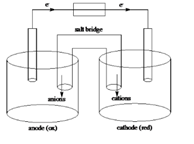
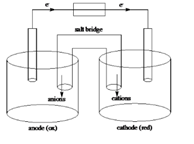
Below is a sketch of a galvanic cell where electrons flow from anode to cathode with a salt bridge between two to supply the required ions and complete the circuit. Cations will always flow to the anode and anions will always flow to the cathode.
By examining oxidation number of atoms in the reaction determine which compound is oxidized and which being reduced.
In above reaction Cr has +3 oxidation state and Cl has 0 oxidation state and in products Cr has +6 oxidation state and Cl has -1 oxidation state. Oxidation number increased for Cr, it is oxidized. This means that
Determine actual half reactions that have occurred.
Cr has lost three electrons because Cr atom has gone from +3 to +6. Add six electrons to the products. The half reaction is now change balanced as well.
It has been converted to
Each Cl atom required one electron because they have gone from 0 to -1, thus add two electrons in reactants for a change balance.
Add two half reactions together and cancel out the electrons to determine overall process. Because Cr half reaction has six electrons multiply Cl half reaction by three before adding two together.
Determine the standard reduction potential from reference table to calculate
Add these values together to give
(b)
Interpretation: The galvanic cells based on given overall reaction needs to be sketched and the value of
Concept Introduction: A galvanic cell is an electro chemical cell that drives electrical energy from chemical reactions taking place within a particular cell.
In a galvanic cell the oxidation and the reduction half cells are separated by a connecting wire so that electrons can flow through the wire.
(b)
Answer to Problem 19E
Sketch of a galvanic cell is as follows:
Values of
Explanation of Solution
By examining oxidation number of atoms in the reaction determine which compound is oxidized and which being reduced.
In above reaction Cu has +2 oxidation state and Mg has 0 oxidation state and in products Cu has 0 oxidation state and Mg has +2oxidation state. Oxidation number increased for Mg, it is oxidized. This means that Mg is at the anode. For Cu oxidation number decreased, it was reduced. This means that
Add two half reactions together and cancel out electrons determine overall process.
Determine the standard reduction potential from reference table to calculate
Add these values together to give
(c)
Interpretation: The galvanic cells based on given overall reaction needs to be sketched and the value of
Concept Introduction: A galvanic cell is an electro chemical cell that drives electrical energy from chemical reactions taking place within a particular cell.
In a galvanic cell the oxidation and the reduction half cells are separated by a connecting wire so that electrons can flow through the wire.
(c)
Answer to Problem 19E
Sketch of a galvanic cell is as follows:
Values of
Explanation of Solution
By examining oxidation number of atoms in the reaction determine which compound is oxidized and which being reduced.
In above reaction I has +5 oxidation state and Fe has +2 oxidation state and in products I has 0 oxidation state and Fe has +3oxidation state. Oxidation number increased for Fe, it is oxidized. This means that
Determine actual half reactions that have occurred
Each I atom has gone from +5 to 0 oxidation state, thus it has gained five electrons. Add ten electrons to reactants. Half-reaction is now charge balanced as well.
To determine overall process add two half reactions together and cancel out electrons. Because I half-reaction has ten electrons, multiply Fe half-reaction by ten before adding two together.
Determine standard reduction potentials from reference table to calculate
Add these values together to give
(d)
Interpretation: The galvanic cells based on given overall reaction needs to be sketched and the value of
Concept Introduction: A galvanic cell is an electro chemical cell that drives electrical energy from chemical reactions taking place within a particular cell.
In a galvanic cell the oxidation and the reduction half cells are separated by a connecting wire so that electrons can flow through the wire.
(d)
Answer to Problem 19E
Sketch of a galvanic cell is as follows:
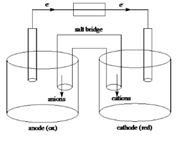
Values of
Explanation of Solution
By examining oxidation number of atoms in the reaction determines which compound is oxidized and which being reduced.
In above reaction Ag has +1 oxidation state and Zn has 0 oxidation state and in products Ag has 0 oxidation state and Zn has +2 oxidation state. This means Zn is at anode. Because oxidation number decrease for Ag. It is reduced. This means that
Determine actual-half reactions that have occurred. Zn has only been converted to
Zn loses two electrons so add two electrons to products to charge balance half-reaction.
Determine standard reduction potentials from reference table to calculate
Add these values together to give
Want to see more full solutions like this?
Chapter 11 Solutions
Chemical Principles
- please provide the structure for this problem, thank you!arrow_forwardDraw the Fischer projection from the skeletal structure shown below. HO OH OH OH OH H Q Drawing Atoms, Bonds and Rings Charges I ☐ T HO H H OH HO I CH2OH H OH Drag H OH -CH2OH CHO -COOH Undo Reset Remove Donearrow_forwardplease provide the structure for this problem, thank youarrow_forward
- presented by Morallen Lig Intermine the hand product for the given mution by adding atoms, bonds, nonhonding diarion panda скуль Step 3: Comp the draw the product Step 2: Agama workup Compithe 429 ملولةarrow_forwardReaction A 0,0arrow_forwardpresented by Morillon Leaning Predict the organic product for the min кусур HSC Adithane carved arnown to come than that to the condon slchroruis in acid in in aquishri with ноюarrow_forward
- 6.15PM Sun Mar 30 K Draw the major product of this reaction. Include any relevant stereochemistry. Ignore inorganic byproducts. Problem 1 of O H [PhзPCH2CH3]*C|¯ NaH Drawing > Q Atoms, Bonds and Draw or tap a nearrow_forward8:17 PM Sun Mar 30 Draw the major product of this reaction. Ignore inorganic byproducts. HSCH2CH2CH2SH, BF3 Probler Drawing Ato Bonds Clarrow_forwardpresented by Mr L How the coprion. (Il Done in no wraction, dew the starting redential) доarrow_forward
- 8:16 PM Sun Mar 30 K Draw the major product of this reaction. Ignore inorganic byproducts. Proble 1. CH3MgBr 2. H3O+ F Drawingarrow_forwardо но оarrow_forwardName the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





