
Concept explainers
(a)
Interpretation:
The reaction and the alcohol that is used for the preparation of ethanal have to be given.
Concept Introduction:
The oxidation of a primary alcohol produces an aldehyde.
The generalized equation is written as,
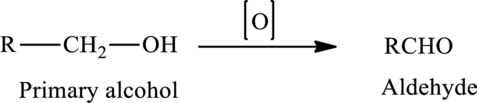
The oxidation of a secondary alcohol produces a ketone.
The generalized equation is written as,
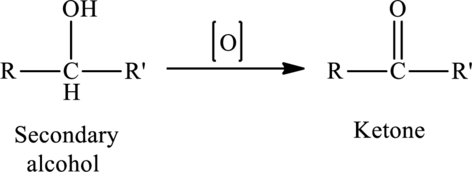
(b)
Interpretation:
The reaction and the alcohol that is used for the preparation of 2-octanone have to be given.
Concept Introduction:
Refer to part (a).
(c)
Interpretation:
The reaction and the alcohol that is used for the preparation of 5-methyloctanal have to be given.
Concept Introduction:
Refer to part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
ACHIEVE/CHEMICAL PRINCIPLES ACCESS 2TERM
- Steps and explanations pleasearrow_forwardUse diagram to answer the following: 1.Is the overall rxn endo- or exothermic. Explain briefly your answer____________________2. How many steps in this mechanism?_____________3. Which is the rate determining step? Explain briefly your answer____________________4. Identify (circle and label) the reactants,the products and intermediate (Is a Cation, Anion, or a Radical?) Please explain and provide full understanding.arrow_forwardDraw the entire mechanism and add Curved Arrows to show clearly how electrons areredistributed in the process. Please explain and provide steps clearly.arrow_forward
- Match the denticity to the ligand. Water monodentate ✓ C₂O2 bidentate H₂NCH₂NHCH2NH2 bidentate x EDTA hexadentate Question 12 Partially correct Mark 2 out of 2 Flag question Provide the required information for the coordination compound shown below: Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2✔ Geometry: linear Oxidation state of transition metal ion: +3 x in 12 correct out of 2 question Provide the required information for the coordination compound shown below. Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2 Geometry: linear 0 Oxidation state of transition metal ion: +3Xarrow_forwardCan you explain step by step behind what the synthetic strategy would be?arrow_forwardPlease explain step by step in detail the reasoning behind this problem/approach/and answer. thank you!arrow_forward
- 2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is present. .OH HO H₂N OHarrow_forwardconsider the rate of the reaction below to be r. Whats the rate after each reaction? Br + NaCN CN + NaBr a. Double the concentration of alkyl bromide b. Halve the concentration of the electrophile & triple concentration of cyanide c. Halve the concentration of alkyl chloridearrow_forwardPredict the organic reactant that is involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





