
Concept explainers
(a)
Interpretation:
The line structure of
Concept Introduction:
Line structure: Line structure is a simplest representation of a hydrocarbon. In line structure, the chain of carbon atoms is shown as a zigzag line. The end of each short line in the zigzag represents a carbon atom. Since the carbon nearly always has a valence of four in organic compounds, it is not necessary to show the hydrogen atoms.
Class of hydrocarbons:
(a)
Answer to Problem 11A.1E
The line structure of

Explanation of Solution
The given condensed formula is

The presence of triple bond in the compound shows that it belongs to alkyne.
(b)
Interpretation:
The line structure of
Concept Introduction:
Refer to part (a).
(b)
Answer to Problem 11A.1E
The line structure of

Explanation of Solution
The given condensed formula is

The presence of single bond in the compound shows that it belongs to alkane.
(c)
Interpretation:
The line structure of
Concept Introduction:
Refer to part (a).
(c)
Answer to Problem 11A.1E
The line structure of

Explanation of Solution
The given condensed formula is

The presence of double bond in the compound shows that it belongs to alkene.
(d)
Interpretation:
The line structure of
Concept Introduction:
Refer to part (a).
(d)
Answer to Problem 11A.1E
The line structure of
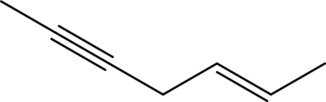
In
Explanation of Solution
The given condensed formula is
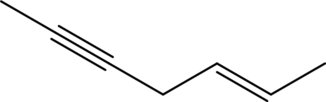
In
(e)
Interpretation:
The line structure of
Concept Introduction:
Refer to part (a).
(e)
Answer to Problem 11A.1E
The line structure of

Explanation of Solution
The given condensed formula is

The presence of double bonds in the compound shows that it belongs to alkene.
Want to see more full solutions like this?
Chapter 11 Solutions
ACHIEVE/CHEMICAL PRINCIPLES ACCESS 2TERM
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

