
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
5th Edition
ISBN: 9781260170405
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11, Problem 11.52P
Devise a synthesis of each compound using
a.  c.
c. 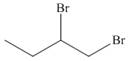 e.
e. 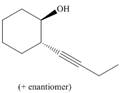
b. 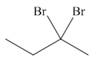 d.
d. 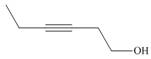
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw a Lewis structure for each of the following species. Again, assign
charges where appropriate.
a. H-H¯
b. CH3-CH3
c. CH3+CH3
d. CH3 CH3
e. CH3NH3+CH3NH3
f. CH30-CH3O¯
g. CH2CH2
-
h. HC2-(HCC) HC2 (HCC)
i. H202×(HOOH) H₂O₂ (HOOH)
Norton
Is molecule 6 an enantiomer?
Show work. Don't give Ai generated solution
Chapter 11 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
Ch. 11 - Problem 11.1 Neopheliosyne B is a novel acetylenic...Ch. 11 - Give the IUPAC name for each compound.Ch. 11 - Give the structures corresponding to each of the...Ch. 11 - Prob. 11.4PCh. 11 - Prob. 11.5PCh. 11 - Which bases can deprotonate acetylene? The pKa...Ch. 11 - Draw the organic products formed when each alkyne...Ch. 11 - Draw additional resonance structures for each...Ch. 11 - Problem 11.9 Draw the products formed when is...Ch. 11 - Explain the following result. Although alkenes...
Ch. 11 - Problem 11.11 Draw the keto tautomer of each...Ch. 11 - Prob. 11.12PCh. 11 - a Draw two different enol tautomers of...Ch. 11 - Prob. 11.14PCh. 11 - Problem 11.15 Draw the organic products formed in...Ch. 11 - Problem 11.16 What acetylide anion and alkyl...Ch. 11 - Problem. 11.17 Show how , and can be used to...Ch. 11 - Prob. 11.18PCh. 11 - Draw the products of each reaction. a. b.Ch. 11 - Prob. 11.20PCh. 11 - Problem 11.21 Use retrosynthetic analysis to show...Ch. 11 - Prob. 11.22PCh. 11 - Give the IUPAC name for each compound. a. b.Ch. 11 - Prob. 11.24PCh. 11 - 11.25 Answer the following questions about...Ch. 11 - 11.26 Give the IUPAC name for each alkyne.
a. ...Ch. 11 - Prob. 11.27PCh. 11 - Which of the following pairs of compounds...Ch. 11 - Prob. 11.29PCh. 11 - 11.30 How is each compound related to A? Choose...Ch. 11 - Prob. 11.31PCh. 11 - Prob. 11.32PCh. 11 - 11.33 Draw the products formed when is treated...Ch. 11 - What reagents are needed to convert (CH3CH2)3CCCH...Ch. 11 - Prob. 11.35PCh. 11 - 11.36 What alkynes give each of the following...Ch. 11 - 11.37 What alkyne gives each compound as the only...Ch. 11 - 11.38 Draw the organic products formed in each...Ch. 11 - 11.39 Draw the structure of compounds A-E in the...Ch. 11 - Prob. 11.40PCh. 11 - Prob. 11.41PCh. 11 - 11.42 What reactions are needed to convert alcohol...Ch. 11 - Prob. 11.43PCh. 11 - Prob. 11.44PCh. 11 - 11.45 Explain the following statement. Although ...Ch. 11 - 11.46 Tautomerization in base resembles...Ch. 11 - 11.47 Draw a stepwise mechanism for each...Ch. 11 - Prob. 11.48PCh. 11 - Prob. 11.49PCh. 11 - 11.50 What acetylide anion and alkyl halide are...Ch. 11 - 11.51 Synthesize each compound from acetylene. You...Ch. 11 - 11.52 Devise a synthesis of each compound using ...Ch. 11 - Prob. 11.53PCh. 11 - Prob. 11.54PCh. 11 - 11.55 Devise a synthesis of the ketone, , from ...Ch. 11 - 11.56 Devise a synthesis of each compound using ...Ch. 11 - Prob. 11.57PCh. 11 - Prob. 11.58PCh. 11 - 11.59 N-Chlorosuccinimide (NCS) serves as a source...Ch. 11 - 11.60 Draw a stepwise mechanism for the following...Ch. 11 - 11.61 Draw a stepwise mechanism for the following...Ch. 11 - Prob. 11.62PCh. 11 - 11.63 Write a stepwise mechanism for each of the...Ch. 11 - Prob. 11.64PCh. 11 - 11.65 Explain why an optically active solution of ...
Additional Science Textbook Solutions
Find more solutions based on key concepts
1. Rub your hands together vigorously. What happens? Discuss the energy transfers and transformations that take...
College Physics: A Strategic Approach (3rd Edition)
Gregor Mendel never saw a gene, yet he concluded that some inherited factors were responsible for the patterns ...
Campbell Essential Biology (7th Edition)
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
To test your knowledge, discuss the following topics with a study partner or in writing ideally from memory. Th...
HUMAN ANATOMY
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
Give the IUPAC name for each compound.
Organic Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 ----||| Molecule 4 Molecule 5 Molecule 6 none of the above mm..arrow_forwardShow work. don't give Ai generated solutionarrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 ----||| Molecule 4 Molecule 5 Molecule 6 none of the above mm..arrow_forward
- Use the vapor-liquid equilibrium data at 1.0 atm. for methanol-water (Table 2-8 ) for the following: If the methanol vapor mole fraction is 0.600, what is the methanol liquid mole fraction? Is there an azeotrope in the methanol-water system at a pressure of 1.0 atmospheres? If water liquid mole fraction is 0.350, what is the water vapor mole fraction? What are the K values of methanol and of water at a methanol mole fraction in the liquid of 0.200? What is the relative volatility αM-W at a methanol mole fraction in the liquid of 0.200?arrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. || |II***** Molecule 1 | Molecule 4 none of the above Molecule 2 Molecule 3 Х mm... C ---||| *** Molecule 5 Molecule 6arrow_forwardis SiBr4 Silicon (IV) tetra Bromine? is KClO2 potassium dihypochlorite ?arrow_forward
- "יוון HO" Br CI Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 Br Br Br HO OH H CI OH ✓ Molecule 4 Molecule 5 Molecule 6 CI Br יייון H Br OH OH CI Br ☐ none of the above × Garrow_forwardUS2 Would this be Uranium (II) diSulfide?arrow_forwardnomenclature for PU(SO4)3arrow_forward
- Li2CrO4 is this Lithium (II) Chromatearrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. NH ** Molecule 1 NH Molecule 4 none of the above Х Molecule 3 Molecule 2 H N wwwwww.. HN Molecule 5 Molecule 6 HN R mw... N H ☐arrow_forwardNomenclature P4S3 Would this be tetraphsophorus tri sulfide?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY