
Concept explainers
(a)
Interpretation: The product that is formed by the reaction is to be drawn.
Concept introduction: The addition of an electrophile to an
The addition of a halogen to an alkyne chain leads to the formation of corresponding
Answer to Problem 11.43P
The product that is formed by the reaction is
Explanation of Solution
The reaction of

Figure 1
The reaction of
The product that is formed by the reaction is
(b)
Interpretation: The product that is formed by the reaction is to be drawn.
Concept introduction: The addition of an electrophile to an alkyne is followed by Markovnikoff’s rule and anti-stereoselectivity.
The addition of a halogen to an alkyne chain leads to the formation of corresponding alkene or alkane. The reaction which includes the addition of bromine atoms to the alkyne chain is known as bromination. Bromination can be done by using the reagents like
Answer to Problem 11.43P
The product that is formed by the reaction is
Explanation of Solution
The reaction of
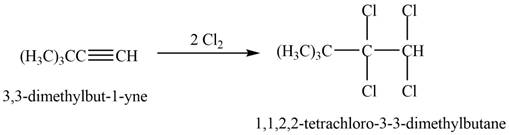
Figure 2
The reaction of
The product that is formed by the reaction is
(c)
Interpretation: The product that is formed by the reaction is to be drawn.
Concept introduction: The addition of a halogen to an alkyne chain leads to the formation of corresponding alkene or alkane. The reaction which includes the addition of bromine atoms to the alkyne chain is known as bromination.
The replacement or substitution of one
Answer to Problem 11.43P
The product that is formed by the reaction is
Explanation of Solution
The reaction of
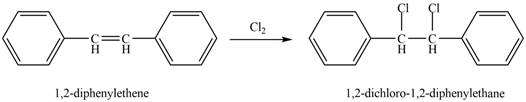
Figure 3
The reaction of
The reaction of
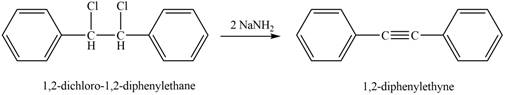
Figure 4
The reaction of
The product that is formed by the reaction is
(d)
Interpretation: The product that is formed by the reaction is to be drawn.
Concept introduction: A stepwise procedure of transforming an alkyne into a carbonyl group is known hydroboration-oxidation reaction. In a hydroboration-oxidation reaction, a terminal alkyne reacts with
Answer to Problem 11.43P
The product that is formed by the reaction is heptanal.
Explanation of Solution
The reaction of
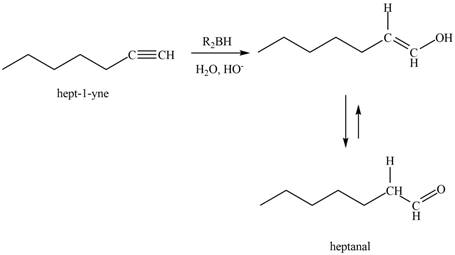
Figure 5
The reaction of
The product that is formed by the reaction is heptanal.
(e)
Interpretation: The product that is formed by the reaction is to be drawn.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The nucleophilic reaction that consists of bimolecular as well as bond-making and bond-breaking steps is termed as
Answer to Problem 11.43P
The product that is formed by the reaction is deuteroacetylene.
Explanation of Solution
The reaction of
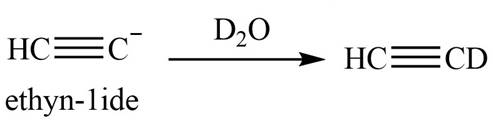
Figure 6
The reaction of
The product that is formed by the reaction is deuteroacetylene.
(f)
Interpretation: The product that is formed by the reaction is to be drawn.
Concept introduction: A terminal alkyne reacts with
Answer to Problem 11.43P
The product that is formed by the reaction is
Explanation of Solution
The reaction of
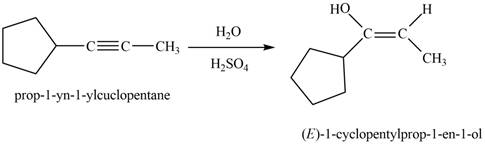
Figure 7
The reaction of
The product that is formed by the reaction is
(g)
Interpretation: The product that is formed by the reaction is to be drawn.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The nucleophilic reaction that consists of bimolecular as well as bond-making and bond-breaking steps is termed as
Answer to Problem 11.43P
The product that is formed by the reaction is
Explanation of Solution
The reaction of
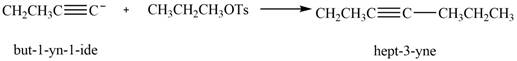
Figure 8
The reaction of
The product that is formed by the reaction is
(h)
Interpretation: The product that is formed by the reaction is to be drawn.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The nucleophilic reaction that consists of bimolecular as well as bond-making and bond-breaking steps is termed as
Answer to Problem 11.43P
The product that is formed by the reaction is
Explanation of Solution
The reaction of
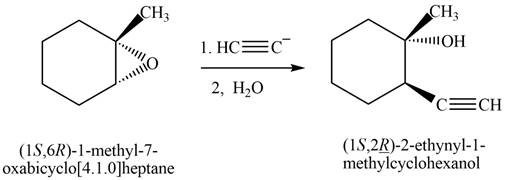
Figure 9
The reaction of
The product that is formed by the reaction is
(i)
Interpretation: The product that is formed by the reaction is to be drawn.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The nucleophilic reaction that consists of bimolecular as well as bond-making and bond-breaking steps is termed as
Answer to Problem 11.43P
The product that is formed by the reaction is
Explanation of Solution
The reaction of
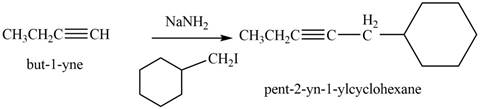
Figure 10
The reaction of
The product that is formed by the reaction is
(j)
Interpretation: The product that is formed by the reaction is to be drawn.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The nucleophilic reaction that consists of bimolecular as well as bond-making and bond-breaking steps is termed as
Answer to Problem 11.43P
The product that is formed by the reaction is
Explanation of Solution
The reaction of ethynylbenzene with
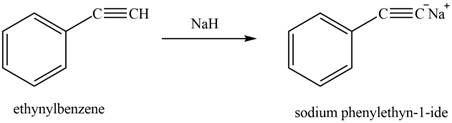
Figure 11
The reaction of ethynylbenzene with
The reaction of
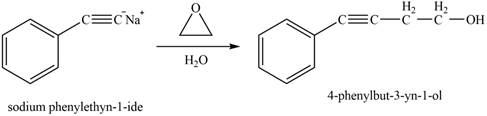
Figure 12
The reaction of
The product that is formed by the reaction is
Want to see more full solutions like this?
Chapter 11 Solutions
Organic Chemistry-Package(Custom)
- Please help me solve this reaction.arrow_forwardIndicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


