
(a)
Interpretation:
For the given reaction, a complete, detailed mechanism is to be drawn, and the major product, ignoring stereochemistry, is to be predicted.
Concept introduction:
An electrophilic addition reaction is an addition reaction where a
Answer to Problem 11.1P
The complete mechanism for the given reaction is
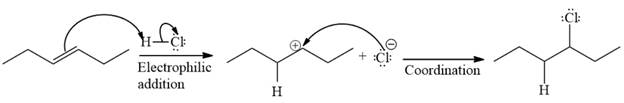
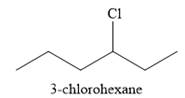
The major product of the reaction is
Explanation of Solution
The given reaction is
![]()
The given reaction occurs in two steps. The electrophilic addition is the first elementary step. The
The product of an electrophilic addition reaction is formed as a result of the addition of the two parts of a Bronsted acid across a double bond, replacing one
(b)
Interpretation:
For the given reaction, the complete, detailed mechanism is to be drawn, and the major product is to be predicted.
Concept introduction:
An electrophilic addition reaction is an addition reaction where a
Answer to Problem 11.1P
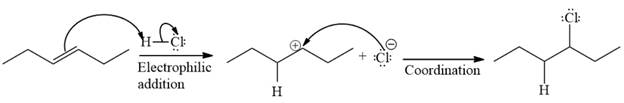
The complete mechanism of the given reaction is
The major product of the reaction is
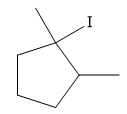
Explanation of Solution
The given reaction is
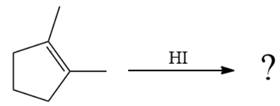
The given reaction occurs in two steps. The first elementary step is an electrophilic addition, where the
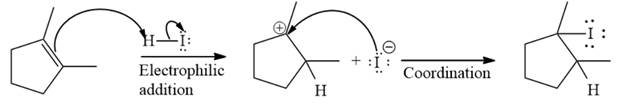
The product of an electrophilic addition reaction is formed as a result of the addition of the two parts of a Bronsted acid across a double bond, replacing one
(c)
Interpretation:
For the given reaction, the complete, detailed mechanism is to be drawn, and the major product is to be predicted.
Concept introduction:
An electrophilic addition reaction is an addition reaction where a
Answer to Problem 11.1P
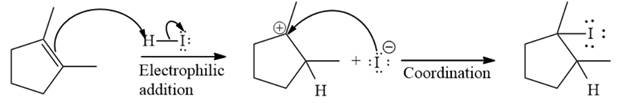
The complete mechanism of the given addition reaction is
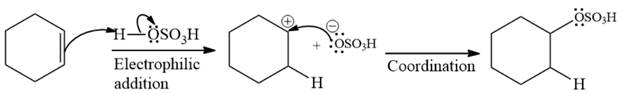
The major product of the reaction is
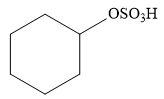
Explanation of Solution
The given addition reaction is
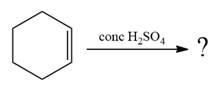
The given reaction occurs in two steps. The electrophilic addition is the first elementary step. The

The product of an electrophilic addition reaction is formed as a result of the addition of the two parts of a Bronsted acid across a double bond, replacing one
(d)
Interpretation:
For the given reaction, the complete, detailed mechanism is to be drawn, and the major product is to be predicted.
Concept introduction:
An electrophilic addition reaction is an addition reaction where a
Answer to Problem 11.1P
The complete mechanism of the given addition reaction is
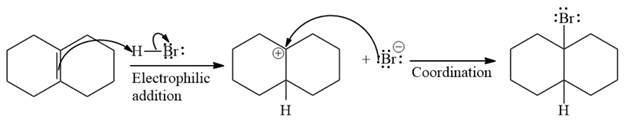
The major product of the reaction is
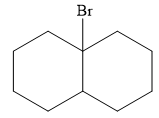
Explanation of Solution
The given addition reaction is
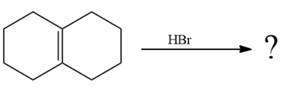
The given reaction occurs in two steps. The electrophilic addition is the first elementary step. The
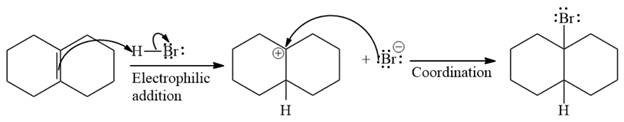
The product of an electrophilic addition reaction is formed as a result of the addition of the two parts of a Bronsted acid across a double bond, replacing one
Want to see more full solutions like this?
Chapter 11 Solutions
Get Ready for Organic Chemistry
- How many chiral centers are there in the following molecule? HO 0 1 ○ 2 ♡ 4 'N'arrow_forwardThe following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
