
a)
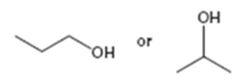
Interpretation:
Among the two alcohols, 1-propanol and 2-propanol, which will react faster with HX to form the corresponding
Concept introduction:
Alcohols react with HX to yield the corresponding alkyl halide. The order of reactivity is methyl < primary < secondary < tertiary.
To state:
Among the two alcohols, 1-propanol and 2-propanol, which will react faster with HX to form the corresponding alkyl halide.
b)
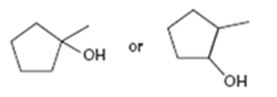
Interpretation:
Among the two alcohols, 1-methylcyclopentanol and 2-methylcyclopentanol, which will react faster with HX to form the corresponding alkyl halide is to be stated.
Concept introduction:
Alcohols react with HX to yield the corresponding alkyl halide. The order of reactivity is methyl < primary < secondary < tertiary.
To state:
Among the two alcohols, 1-methylcyclopentanol and 2-methylcyclopentanol, which will react faster with HX to form the corresponding alkyl halide.
c)
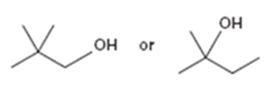
Interpretation:
Among the two alcohols, 2,2-dimethyl-1-propanol and 2-methyl-2-butanol, which will react faster with HX to form the corresponding alkyl halide is to be stated.
Concept introduction:
Alcohols react with HX to yield the corresponding alkyl halide. The order of reactivity is methyl < primary < secondary < tertiary.
To state:
Among the two alcohols, 2,2-dimethyl-1-propanol and 2-methyl-2-butanol, which will react faster with HX to form the corresponding alkyl halide.
Trending nowThis is a popular solution!

Chapter 10 Solutions
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
- Can the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? ? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C :0 T Add/Remove step Garrow_forwardThe following equations represent the formation of compound MX. What is the AH for the electron affinity of X (g)? X₂ (g) → 2X (g) M (s) → M (g) M (g) M (g) + e- AH = 60 kJ/mol AH = 22 kJ/mol X (g) + e-X (g) M* (g) +X (g) → MX (s) AH = 118 kJ/mol AH = ? AH = -190 kJ/mol AH = -100 kJ/mol a) -80 kJ b) -30 kJ c) -20 kJ d) 20 kJ e) 156 kJarrow_forwardA covalent bond is the result of the a) b) c) d) e) overlap of two half-filled s orbitals overlap of a half-filled s orbital and a half-filled p orbital overlap of two half-filled p orbitals along their axes parallel overlap of two half-filled parallel p orbitals all of the abovearrow_forward
- Can the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C T Add/Remove step X ноarrow_forwardWhich one of the following atoms should have the largest electron affinity? a) b) c) d) 으으 e) 1s² 2s² 2p6 3s¹ 1s² 2s² 2p5 1s² 2s² 2p 3s² 3p² 1s² 2s 2p 3s² 3p6 4s2 3ds 1s² 2s² 2p6arrow_forwardAll of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forward
- A student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forwardPredict the major products of this organic reaction:arrow_forwardCalculate the density of 21.12 g of an object that displaces 0.0250 L of water.arrow_forward
- Draw the expected reactant R28. Cu(II) CO₂Mearrow_forwardPpplllleeeaaasssseeee helllppp wiithhh thisss Organic chemistryyyyyy I talked like this because AI is very annoyingarrow_forwardName the family to which each organic compound belongs. The first answer has been filled in for you. compound CH₂ || CH3-C-NH2 0 ။ CH3-C-CH₂ CH=O–CH=CH, CH₂ HO CH2-CH2-CH-CH3 family amine Darrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


