
Concept explainers
a)
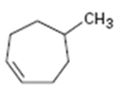
Interpretation:
The product expected when 5-methylcycloheptene reacts with NBS is to be shown. If more than one product is possible the structures of all of them are to be shown.
Concept introduction:
NBS is used mainly for allylic bromination. An unsymmetrical
To show:
The structures of all the products expected when 5-methylcycloheptene reacts with NBS.
b)
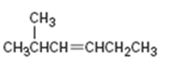
Interpretation:
The product expected when 2-methyl-3-hexene reacts with NBS is to be shown. If more than one product is possible the structures of all of them are to be shown.
Concept introduction:
NBS is used mainly for allylic bromination. An unsymmetrical alkene will lead to a mixture of products as two different allyl radicals will be produced as a result of delocalization. The two products are not formed in equal amounts because the intermediate allylic radical is not symmetrical and the reaction at two ends will not take place to the same extent. Reaction at less hindered primary side is more favored.
To show:
The structures of all the products expected when 2-methyl-3-hexene reacts with NBS.
Trending nowThis is a popular solution!

Chapter 10 Solutions
Organic Chemistry - With Access (Custom)
- A 2-step reaction has the following mechanism: | 1. (fast) R2 R+R 2. (slow) R+Q K₂ P k_1 What series does it have? (A). v= - = (k + k1 − k-1)[R2][Q] (B). v=-k₁[R₂] + k₁[R]² - k₂[R][Q] (C). v=k₂[R]²[Q]² (D). v = k[R₂]1/2[Q]arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardLabel the α and ẞ carbons in each alkyl halide. Draw all possible elimination products formed when each alkyl halide is treated with K-OC(CH3), b. ان Brarrow_forwardSuppose a reaction has the following mechanism:A + B → C + D C + C → F F + B → A + A + GIt is known that C is a reaction intermediate. Of the following options, indicate which are true:1. The overall reaction could be 3B → 2D + G.2. A could be a catalyst.3. C is the only intermediate that can exist.arrow_forward
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

