
Principles of General, Organic, Biological Chemistry
2nd Edition
ISBN: 9780073511191
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10.4, Problem 10.7P
Identify the
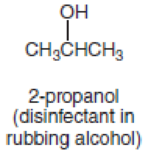
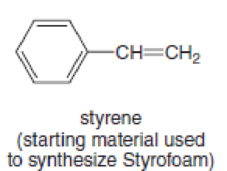
- a. H2NCH2CH2CH2CH2NH2
Putrescine (putrid odor of rotting fish)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In the analysis of Mg content in a 25 mL sample, a titration volume of 5 mL was obtained using 0.01 M EDTA. Calculate the Mg content in the sample if the Ca content is 20 ppm
Predict the organic products that form in the reaction below:
H.
H+
+
OH
H+
Y
Note: You may assume you have an excess of either reactant if the reaction requires more than one of those molecules to form the
products.
In the drawing area below, draw the skeletal ("line") structures of the missing organic products X and Y. You may draw the
structures in any arrangement that you like, so long as they aren't touching.
Explanation
Check
Click and drag to start drawing a
structure.
G
X
C
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Access
+
111
Carbonyl Chem
Choosing reagants for a Wittig reaction
What would be the best choices for the missing reagents 1 and 3 in this synthesis?
1. PPh3
3
1
2
2. n-BuLi
• Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like.
Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is.
• Note: if one of your reagents needs to contain a halogen, use bromine.
Explanation
Check
Click and drag to start drawing a structure.
×
©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use
Chapter 10 Solutions
Principles of General, Organic, Biological Chemistry
Ch. 10.1 - Prob. 10.1PCh. 10.2 - Fill in all Hs and lone pairs in each compound. a....Ch. 10.3 - Convert each compound to a condensed formula.Ch. 10.3 - Convert each condensed formula to a complete...Ch. 10.3 - Convert each skeletal structure to a complete...Ch. 10.3 - How many Hs are bonded to each indicated carbon...Ch. 10.4 - Identify the functional groups in each compound....Ch. 10.4 - For each compound: [1] Identify the functional...Ch. 10.4 - Prob. 10.9PCh. 10.4 - Prob. 10.10P
Ch. 10.4 - Convert the ball-and-stick model of the local...Ch. 10.5 - How many hydrogen atoms are present in each...Ch. 10.5 - Prob. 10.13PCh. 10.5 - Prob. 10.14PCh. 10.5 - Prob. 10.15PCh. 10.5 - Prob. 10.16PCh. 10.6 - Give the IUPAC name for each compound.Ch. 10.6 - Prob. 10.18PCh. 10.6 - Prob. 10.19PCh. 10.6 - Prob. 10.20PCh. 10.7 - Prob. 10.21PCh. 10.7 - Prob. 10.22PCh. 10.9 - Answer the following questions about pentane...Ch. 10.9 - Prob. 10.24PCh. 10.9 - Prob. 10.25PCh. 10.10 - Prob. 10.26PCh. 10 - Prob. 10.27UKCCh. 10 - Prob. 10.28UKCCh. 10 - Prob. 10.29UKCCh. 10 - Prob. 10.30UKCCh. 10 - Prob. 10.31UKCCh. 10 - The largest known cycloalkane with a single ring...Ch. 10 - Draw three constitutional isomers having molecular...Ch. 10 - Draw four constitutional isomers having molecular...Ch. 10 - Answer the following questions about the alkane...Ch. 10 - Answer the questions in Problem 10.35 for the...Ch. 10 - Prob. 10.37UKCCh. 10 - Procaine (trade name Novocain) is a local...Ch. 10 - Prob. 10.39APCh. 10 - Prob. 10.40APCh. 10 - Complete each structure by filling in all Hs and...Ch. 10 - Prob. 10.42APCh. 10 - Prob. 10.43APCh. 10 - Prob. 10.44APCh. 10 - Prob. 10.45APCh. 10 - Prob. 10.46APCh. 10 - Convert each compound to a condensed structure.Ch. 10 - Prob. 10.48APCh. 10 - Prob. 10.49APCh. 10 - Prob. 10.50APCh. 10 - Prob. 10.51APCh. 10 - Prob. 10.52APCh. 10 - Albuterol (trade names: Proventil and Ventolin) is...Ch. 10 - Prob. 10.54APCh. 10 - Prob. 10.55APCh. 10 - Prob. 10.56APCh. 10 - Prob. 10.57APCh. 10 - Prob. 10.58APCh. 10 - Prob. 10.59APCh. 10 - Prob. 10.60APCh. 10 - Prob. 10.61APCh. 10 - Prob. 10.62APCh. 10 - Prob. 10.63APCh. 10 - Prob. 10.64APCh. 10 - Prob. 10.65APCh. 10 - Prob. 10.66APCh. 10 - Prob. 10.67APCh. 10 - Prob. 10.68APCh. 10 - Prob. 10.69APCh. 10 - Prob. 10.70APCh. 10 - Give the IUPAC name for each compound.Ch. 10 - Prob. 10.72APCh. 10 - Prob. 10.73APCh. 10 - Prob. 10.74APCh. 10 - Prob. 10.75APCh. 10 - Prob. 10.76APCh. 10 - Give the structure corresponding to each IUPAC...Ch. 10 - Prob. 10.78APCh. 10 - Prob. 10.79APCh. 10 - Each of the following IUPAC names is incorrect....Ch. 10 - Prob. 10.81APCh. 10 - Prob. 10.82APCh. 10 - Prob. 10.83APCh. 10 - Prob. 10.84APCh. 10 - Prob. 10.85APCh. 10 - Prob. 10.86APCh. 10 - Write a balanced equation for the incomplete...Ch. 10 - Prob. 10.88APCh. 10 - Prob. 10.89APCh. 10 - Prob. 10.90APCh. 10 - Prob. 10.91APCh. 10 - Prob. 10.92APCh. 10 - Answer the following questions for the cycloalkane...Ch. 10 - Prob. 10.94APCh. 10 - Prob. 10.95CPCh. 10 - Prob. 10.96CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A student proposes the transformation below in one step of an organic synthesis. There may be one or more reactants missing from the left-hand side, but there are no products missing from the right-hand side. There may also be catalysts, small inorganic reagents, and other important reaction conditions missing from the arrow. • Is the student's transformation possible? If not, check the box under the drawing area. . If the student's transformation is possible, then complete the reaction by adding any missing reactants to the left-hand side, and adding required catalysts, inorganic reagents, or other important reaction conditions above and below the arrow. • You do not need to balance the reaction, but be sure every important organic reactant or product is shown. + T X O O лет-ле HO OH HO OH This transformation can't be done in one step.arrow_forwardDetermine the structures of the missing organic molecules in the following reaction: X+H₂O H* H+ Y OH OH Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structures of the missing organic molecules X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. X Sarrow_forwardPredict the major products of this organic reaction. If there aren't any products, because nothing will happen, check the box under the drawing area instead. No reaction. HO. O :☐ + G Na O.H Click and drag to start drawing a structure. XS xs H₂Oarrow_forward
- What are the angles a and b in the actual molecule of which this is a Lewis structure? H H C H- a -H b H Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal groups may have slightly different sizes. a = b = 0 °arrow_forwardWhat are the angles a and b in the actual molecule of which this is a Lewis structure? :0: HCOH a Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal that might be caused by the fact that different electron groups may have slightly different sizes. a = 0 b=0° Sarrow_forwardDetermine the structures of the missing organic molecules in the following reaction: + H₂O +H OH O OH +H OH X Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structure of the missing organic molecule X. Click and drag to start drawing a structure.arrow_forward
- Identify the missing organic reactant in the following reaction: x + x O OH H* + ☑- X H+ O O Х Note: This chemical equation only focuses on the important organic molecules in the reaction. Additional inorganic or small-molecule reactants or products (like H₂O) are not shown. In the drawing area below, draw the skeletal ("line") structure of the missing organic reactant X. Click and drag to start drawing a structure. Carrow_forwardCH3O OH OH O hemiacetal O acetal O neither O 0 O hemiacetal acetal neither OH hemiacetal O acetal O neither CH2 O-CH2-CH3 CH3-C-OH O hemiacetal O acetal CH3-CH2-CH2-0-c-O-CH2-CH2-CH3 O neither HO-CH2 ? 000 Ar Barrow_forwardWhat would be the best choices for the missing reagents 1 and 3 in this synthesis? 1. PPh3 2 2. n-BuLi 3 Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Explanation Check Click and drag to start drawing a structure.arrow_forward
- Predict the products of this organic reaction: NaBH3CN + NH2 ? H+ Click and drag to start drawing a structure. ×arrow_forwardPredict the organic products that form in the reaction below: + OH +H H+ ➤ ☑ X - Y Note: You may assume you have an excess of either reactant if the reaction requires more than one of those molecules to form the products. In the drawing area below, draw the skeletal ("line") structures of the missing organic products X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. Garrow_forwardPredict the organic products that form in the reaction below: OH H+ H+ + ☑ Y Note: You may assume you have an excess of either reactant if the reaction requires more than one of those molecules to form the products. In the drawing area below, draw the skeletal ("line") structures of the missing organic products X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. ✓ marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY