
ORGANIC CHEMISTRY
6th Edition
ISBN: 9781266633973
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10.16, Problem 26P
What alkylborane is formed from hydroboration of each
a 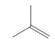 b.
b. 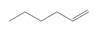 c.
c. 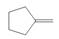
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
If the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate
the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data:
molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.
If the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate
the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data:
molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.
If the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate
the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data:
molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.
Chapter 10 Solutions
ORGANIC CHEMISTRY
Ch. 10.1 - Prob. 1PCh. 10.2 - Problem 10.2 How many degrees of unsaturation are...Ch. 10.3 - Give the IUPAC name for each alkene. abcdeCh. 10.3 - Give the IUPAC name for each polyfunctional...Ch. 10.3 - Prob. 9PCh. 10.6 - Linolenic acidTable 10.2 and stearidonic acid are...Ch. 10.7 - Prob. 12PCh. 10.9 - Problem 10.13 What product is formed when each...Ch. 10.9 - Prob. 14PCh. 10.10 - Problem 10.15 Draw the products formed when each...
Ch. 10.10 - Prob. 16PCh. 10.10 - Prob. 17PCh. 10.10 - Addition of HBr to which of the following alkenes...Ch. 10.11 - Problem 10.19 Draw the products, including...Ch. 10.11 - Prob. 20PCh. 10.12 - Problem 10.21 What two alkenes give rise to each...Ch. 10.12 - Prob. 22PCh. 10.13 - Problem 10.23 Draw the products of each reaction,...Ch. 10.14 - Problem 10.24 Draw all stereoisomers formed in...Ch. 10.15 - Prob. 25PCh. 10.16 - Problem 10.26 What alkylborane is formed from...Ch. 10.16 - Draw the products formed when each alkene is...Ch. 10.16 - What alkene can be used to prepare each alcohol as...Ch. 10.16 - Prob. 29PCh. 10.17 - Draw the products of each reaction using the two...Ch. 10.18 - Problem 10.31 Devise a synthesis of each compound...Ch. 10 - Give the IUPAC name for each compound. a.b.Ch. 10 - a Label the carbon-carbon double bond in A as E or...Ch. 10 - Prob. 34PCh. 10 - 10.35 Calculate the number of degrees of...Ch. 10 - Prob. 36PCh. 10 - Label the alkene in each drug as E or Z....Ch. 10 - Give the IUPAC name for each compound. a. c. e. b....Ch. 10 - Prob. 39PCh. 10 - 10.40 (a) Draw all possible stereoisomers of, and...Ch. 10 - Prob. 41PCh. 10 - 10.42 Now that you have learned how to name...Ch. 10 - Prob. 43PCh. 10 - Prob. 44PCh. 10 - Prob. 45PCh. 10 - Draw the products formed when (CH3)2C=CH2 is...Ch. 10 - What alkene can be used to prepare each alkyl...Ch. 10 - Prob. 48PCh. 10 - Draw the constitutional isomer formed in each...Ch. 10 - Prob. 50PCh. 10 - Draw all stereoisomers formed in each reaction. a....Ch. 10 - Draw the products of each reaction, including...Ch. 10 - Prob. 53PCh. 10 - Draw a stepwise mechanism that shows how all three...Ch. 10 - Less stable alkenes can be isomerized to more...Ch. 10 - Prob. 60PCh. 10 - Prob. 61PCh. 10 - Bromoetherification, the addition of the elements...Ch. 10 - Devise a synthesis of each product from the given...Ch. 10 - 10.65 Draw a synthesis of each compound from...
Additional Science Textbook Solutions
Find more solutions based on key concepts
How does the removal of hydrogen atoms from nutrient molecules result in a loss of energy from the nutrient mol...
SEELEY'S ANATOMY+PHYSIOLOGY
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
Gregor Mendel never saw a gene, yet he concluded that some inherited factors were responsible for the patterns ...
Campbell Essential Biology (7th Edition)
1. Genetics affects many aspects of our lives. Identify three ways genetics affects your life or the life of a ...
Genetic Analysis: An Integrated Approach (3rd Edition)
More than one choice may apply. Using the terms listed below, fill in the blank with the proper term. anterior ...
Essentials of Human Anatomy & Physiology (12th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine the distance between the metal and the OHP layer using the Helm- holtz model when the electrode's differential capacitance is 145 μF cm². DATA: dielectric constant of the medium for the interfacial zone &r= lectric constant of the vacuum &0 = 8.85-10-12 F m-1 = 50, die-arrow_forwardDescribe a sequence of photophysical processes that can be followed by radiation adsorbed by a molecule in the ground state to give rise to phosphorescent emission.arrow_forwardState two similarities between fluorescence and phosphorescence.arrow_forward
- State three photophysical processes that can be related to the effects of incident radiation on a molecule in its ground state. Consider that radiation can give rise to fluorescent emission, but not phosphorescent emission.arrow_forwardIn a photochemical reaction, how is the rate of the process related to its quantum yield?arrow_forwardPrimary and global quantum yields in photochemistry. Define them and give their formulas. Differentiate between them.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License