
Concept explainers
Give the IUPAC name for each compound.
a.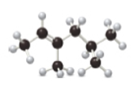 b.
b. 
(a)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 32P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
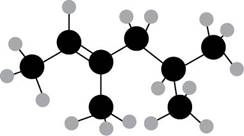
Figure 1
The ball and stick model of the compound is given. The black color ball represents the carbon atom and white color ball represent the hydrogen atom.
The numbering of carbon atoms present in the longest carbon chain is shown below.
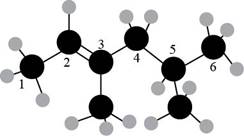
Figure 2
The longest carbon chain has six carbon atoms. The root word used for six carbon atoms is hex and the suffix used for
The IUPAC name for the given compound is
(b)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 32P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
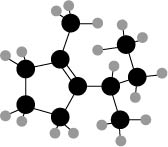
Figure 3
The ball and stick model of the compound is given. The black color ball represents the carbon atom and white color ball represent the hydrogen atom.
The numbering of carbon atoms present in the longest carbon chain is shown below.
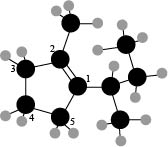
Figure 4
The longest carbon chain has five carbon atoms. The root word used for five carbon atoms is pent and the suffix used for
The IUPAC name for the given compound is
Want to see more full solutions like this?
Chapter 10 Solutions
ORGANIC CHEMISTRY
Additional Science Textbook Solutions
Physics for Scientists and Engineers
Human Physiology: An Integrated Approach (8th Edition)
Anatomy & Physiology (6th Edition)
Human Biology: Concepts and Current Issues (8th Edition)
- What does the phrase 'fit for purpose' mean in relation to analytical chemistry? Please provide examples too.arrow_forwardFor each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects Resonance Effects Overall Electron-Density × NO2 ○ donating O donating O withdrawing O withdrawing O electron-rich electron-deficient no inductive effects O no resonance effects O similar to benzene E [ CI O donating withdrawing O no inductive effects Explanation Check ○ donating withdrawing no resonance effects electron-rich electron-deficient O similar to benzene © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accesarrow_forwardUnderstanding how substituents activate Rank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. Explanation HN NH2 Check X (Choose one) (Choose one) (Choose one) (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Aarrow_forward
- Identifying electron-donating and electron-withdrawing effects on benzene For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Inductive Effects Resonance Effects Overall Electron-Density Molecule CF3 O donating O donating O withdrawing O withdrawing O no inductive effects O no resonance effects electron-rich electron-deficient O similar to benzene CH3 O donating O withdrawing O no inductive effects O donating O withdrawing Ono resonance effects O electron-rich O electron-deficient O similar to benzene Explanation Check Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward* Hint: Think back to Chem 1 solubility rules. Follow Up Questions for Part B 12. What impact do the following disturbances to a system at equilibrium have on k, the rate constant for the forward reaction? Explain. (4 pts) a) Changing the concentration of a reactant or product. (2 pts) b) Changing the temperature of an exothermic reaction. (2 pts) ofarrow_forwardDraw TWO general chemical equation to prepare Symmetrical and non-Symmetrical ethers Draw 1 chemical reaction of an etherarrow_forward
- Give the chemical equation for the preparation of: -Any aldehyde -Any keytonearrow_forward+ C8H16O2 (Fatty acid) + 11 02 → 8 CO2 a. Which of the above are the reactants? b. Which of the above are the products? H2o CO₂ c. Which reactant is the electron donor? Futty acid d. Which reactant is the electron acceptor? e. Which of the product is now reduced? f. Which of the products is now oxidized? 02 #20 102 8 H₂O g. Where was the carbon initially in this chemical reaction and where is it now that it is finished? 2 h. Where were the electrons initially in this chemical reaction and where is it now that it is finished?arrow_forward→ Acetyl-CoA + 3NAD+ + 1FAD + 1ADP 2CO2 + CoA + 3NADH + 1FADH2 + 1ATP a. Which of the above are the reactants? b. Which of the above are the products? c. Which reactant is the electron donor? d. Which reactants are the electron acceptors? e. Which of the products are now reduced? f. Which product is now oxidized? g. Which process was used to produce the ATP? h. Where was the energy initially in this chemical reaction and where is it now that it is finished? i. Where was the carbon initially in this chemical reaction and where is it now that it is finished? j. Where were the electrons initially in this chemical reaction and where is it now that it is finished?arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning


