
(a)
Interpretation:
The major product of given reaction should be given.
Concept introduction:
Hofmann elimination:
Quartnary ammonium ion undergoes limination when using strong base like hydroxide ion this reaction is called as hofmann elimination.
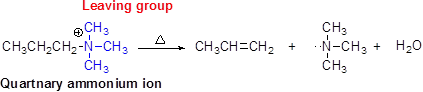
(b)
Interpretation:
The major product of given reaction should be given.
Concept introduction:
Hofmann elimination:
Quartnary ammonium ion undergoes limination when using strong base like hydroxide ion this reaction is called as hofmann elimination.
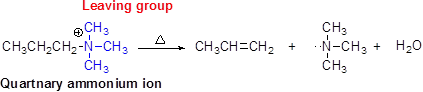
(c)
Interpretation:
The major product of given reaction should be given.
Concept introduction:
Hofmann elimination:
Quaternary ammonium ion undergoes elimination when using strong base like hydroxide ion this reaction is called as Hofmann elimination.
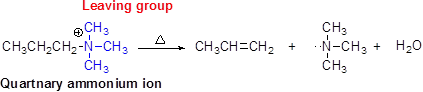
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Organic Chemistry; Organic Chemistry Study Guide A Format: Kit/package/shrinkwrap
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
