
Organic Chemistry; Organic Chemistry Study Guide A Format: Kit/package/shrinkwrap
8th Edition
ISBN: 9780134581064
Author: Bruice, Paula Yurkanis
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Question
Chapter 10.7, Problem 31P
(a)
Interpretation Introduction
Interpretation:
The stereo isomeric product should be given when the
Concept introduction:
An alkene undergoes an oxidation reaction with Osmium tetra oxide and followed by hydrolysis to give a cis 1, 2
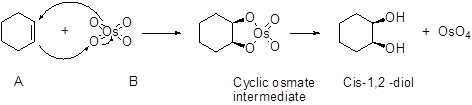
(b)
Interpretation Introduction
Interpretation:
The stereo isomeric product should be given when the alkene reaction with osmium tetraoxide followed by hydrogen peroxide hydrolysis.
Concept introduction:
An alkene undergoes an oxidation reaction with Osmium tetra oxide and followed by hydrolysis to give a cis 1, 2 diol for example,
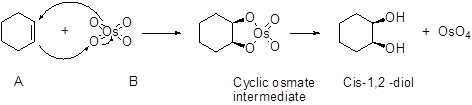
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Redraw the tripeptide with or without its acidic hydrogensto demonstrate where the total charge of -2 comes from: *see image
2. Consider the data below to answer the following questions.
Cyanohydrins are important intermediates in the synthesis of α-hydroxycarboxylic acids from ketones and
aldehydes. The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid.
Nitriles can also be hydrolyzed to carboxylic acids using aqueous base. Unfortunately, when a cyanohydrin
is treated with aqueous base the original carbonyl compound is isolated.
OH
CH-COOH
0
HO CN
C
H30*
C.
H
H
HC N
NaOH
H₂O
C=O
0
cyanohydrin
H
+ NaCN + H₂O
Assign all integrated peaks
Chapter 10 Solutions
Organic Chemistry; Organic Chemistry Study Guide A Format: Kit/package/shrinkwrap
Ch. 10.1 - Why are NH3 and CH3NH2 no longer nucleophiles when...Ch. 10.1 - Explain the difference in reactivity between...Ch. 10.1 - Prob. 5PCh. 10.1 - Prob. 6PCh. 10.1 - Prob. 8PCh. 10.2 - Prob. 9PCh. 10.3 - Prob. 11PCh. 10.3 - Show how 1-propanol can be converted into the...Ch. 10.4 - Which of the following alcohols dehydrates the...Ch. 10.4 - Prob. 14P
Ch. 10.4 - Prob. 15PCh. 10.4 - Propose a mechanism for each of the following...Ch. 10.4 - Draw the product of each of the following...Ch. 10.4 - Explain why the following alcohols, when heated...Ch. 10.4 - What stereoisomers are formed in the following...Ch. 10.4 - Prob. 20PCh. 10.4 - What alcohol would you treat with phosphorus...Ch. 10.5 - Prob. 22PCh. 10.6 - What are the major products obtained when each of...Ch. 10.6 - Prob. 26PCh. 10.7 - Prob. 27PCh. 10.7 - Would you expect the reactivity of a five-membered...Ch. 10.7 - Prob. 29PCh. 10.7 - What products are obtained from the reaction of...Ch. 10.7 - Prob. 31PCh. 10.7 - Prob. 32PCh. 10.7 - Prob. 33PCh. 10.8 - Draw the mechanism for formation of the two...Ch. 10.8 - Prob. 35PCh. 10.8 - Prob. 36PCh. 10.8 - How do the major products obtained from...Ch. 10.8 - Explain why the two arene oxides in Problem 38...Ch. 10.8 - Which compound is more likely to be carcinogenic?Ch. 10.8 - Three arene oxides can be obtained from...Ch. 10.9 - Explain why the half-life (the time it takes for...Ch. 10.10 - Prob. 43PCh. 10.10 - Prob. 44PCh. 10.10 - Prob. 45PCh. 10.10 - Prob. 46PCh. 10.10 - Prob. 47PCh. 10.10 - Describe a synthesis for each of the following...Ch. 10.11 - Using an alkyl halide and a thiol as starting...Ch. 10.11 - The following three nitrogen mustards were studied...Ch. 10.11 - Why is melphalan a good cancer drug?Ch. 10.11 - Prob. 53PCh. 10.12 - Propose a mechanism for the following reaction:Ch. 10 - Prob. 55PCh. 10 - Which compound is more likely to be carcinogenic?Ch. 10 - Prob. 57PCh. 10 - Prob. 58PCh. 10 - When heated with H2SO4, both...Ch. 10 - What is the major product obtained from the...Ch. 10 - Write the appropriate reagent over each arrow.Ch. 10 - What alkenes would you expect to be obtained from...Ch. 10 - Prob. 63PCh. 10 - Prob. 64PCh. 10 - When deuterated phenanthrene oxide undergoes a...Ch. 10 - An unknown alcohol with a molecular formula of...Ch. 10 - Explain why the acid-catalyzed dehydration of an...Ch. 10 - Prob. 68PCh. 10 - Prob. 69PCh. 10 - Propose a mechanism for the following reaction:Ch. 10 - What product would be formed if the four-membered...Ch. 10 - Which of the following ethers would be obtained in...Ch. 10 - Using the given starting material any necessary...Ch. 10 - Prob. 74PCh. 10 - When 3-methyl-2-butanol is heated with...Ch. 10 - Draw structures for compounds AF.Ch. 10 - Propose a mechanism for each of the following...Ch. 10 - How could you synthesize isopropyl propyl ether,...Ch. 10 - When ethyl ether is heated with excess HI for...Ch. 10 - When the following seven-membered ring alcohol is...Ch. 10 - Ethylene oxide reacts readily with HO because of...Ch. 10 - Describe how each of the following compounds could...Ch. 10 - Propose a mechanism for each of the following...Ch. 10 - Triethylene glycol is one of the products obtained...Ch. 10 - Prob. 85PCh. 10 - Propose a mechanism for the following reaction:Ch. 10 - Prob. 87PCh. 10 - An ion with a positively charged nitrogen atom in...Ch. 10 - The following reaction takes place several times...Ch. 10 - Prob. 90PCh. 10 - Propose a mechanism for each of the following...Ch. 10 - A vicinal diol has OH groups on adjacent carbons....Ch. 10 - Prob. 93PCh. 10 - Prob. 94PCh. 10 - Two stereoisomers are obtained from the reaction...Ch. 10 - Propose a mechanism for each or the following...Ch. 10 - Triethylenemelamine (TEM) is an antitumor agent....
Knowledge Booster
Similar questions
- - Consider the data in the Table below to answer the following questions: Acidities of Substituted Benzoic and Acetic Acids pk,s at 25C Y-CH COOH Y Y - CH₂COOH meta para H 4.75 4.19 4.19 2.47 3.64 3.55 3.57 4.09 4.46 CN OCH 3 A. Draw the structure of the strongest acid in the table above. B. Explain why cyanoacetic acid and methoxyacetic acid are more acidic than their correspondingly substituted benzoic acid counterparts.arrow_forwardDraw the curved arrow mechanism for this reaction starting with 2-propanol in sulfuric acid. Show all nonzero formal charges and all nonbonded electrons in each step. Species not involved in a particular step do not need to be included in that step, and resonance forms do not need to be shown. Note that the alcohol is in much higher concentration than H₂O in this reaction. Harrow_forwardProvide reactions showing the following conversions: * see imagearrow_forward
- . Draw structures corresponding to each of the following names or Provide IUPAC names for each of the ollowing structures [for 4 ONLY]. A. 2-propylpentanoic acid. B. m-chlorobenzoic acid. D. C. O O HOC(CH2)3COH glutaricadd OH OH H3C CH3 C=C H COOH salicylicadd tiglicadd CH₂C=N Joe Marrow_forward. Provide structure(s) for the starting material(s), reagent(s) or the major organic product(s) of each of the ollowing reactions or sequences of reactions. Show all relevant stereochemistry [five only] A. O B. OET CH3 1. LIAIH, ether 2 H₂O O (CH3)2CH-C-CI + 0 0 ether (CH3)2CH-C-O-C-CH3 CH3 C. 0 OH HO CH3 ° Clarrow_forwardHow would you prepare each of the following compounds using either an acetoacetic ester synthesis or a alonic ester synthesis? Show all intermediate structures and all reagents.[Three only] A. B. COOH OH C. D. 0 H2C CHCH2CH2CCH3arrow_forward
- Fats and greases have mostly aliphatic regions which are hydrophobic. Provide a schematic of howsoaps/detergents remove fats and grease from the soiled material. * see imagearrow_forwardWhat chemical has the common name "lye"? Pick one of the 3 esters and show the hydrolysis mechanism to make a carboxylic acid. The organic “R” should be used to limit the redrawing time of the entire molecule. * see imagearrow_forwardProvide the products for each reaction. There are two and they are not related. *see imagearrow_forward
- d. a phenylal Give the major organic product(s) of each of the following reactions or sequences of reactions. Show all levant stereochemistry. [three only] 0 A. B. CH3 Bra CH3COOH OH 1. Br₂, PBrz 2 H₂O 12arrow_forward2arrow_forwardShow how the following conversions might be accomplished. Show all reagents and all intermediate ructures. More than one step may be required [2 ONLY]: A. B. ° C. OH 0 OH 0arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
